High gene expression of inflammatory markers and IL-17A correlates with severity of injection site reactions of Atlantic salmon vaccinated with oil-adjuvanted vaccines
- PMID: 20507624
- PMCID: PMC2996971
- DOI: 10.1186/1471-2164-11-336
High gene expression of inflammatory markers and IL-17A correlates with severity of injection site reactions of Atlantic salmon vaccinated with oil-adjuvanted vaccines
Abstract
Background: Two decades after the introduction of oil-based vaccines in the control of bacterial and viral diseases in farmed salmonids, the mechanisms of induced side effects manifested as intra-abdominal granulomas remain unresolved. Side effects have been associated with generation of auto-antibodies and autoimmunity but the underlying profile of inflammatory and immune response has not been characterized. This study was undertaken with the aim to elucidate the inflammatory and immune mechanisms of granuloma formation at gene expression level associated with high and low side effect (granuloma) indices.Groups of Atlantic salmon parr were injected intraperitoneally with oil-adjuvanted vaccines containing either high or low concentrations of Aeromonas salmonicida or Moritella viscosa antigens in order to induce polarized (severe and mild) granulomatous reactions. The established granulomatous reactions were confirmed by gross and histological methods at 3 months post vaccination when responses were known to have matured. The corresponding gene expression patterns in the head kidneys were profiled using salmonid cDNA microarrays followed by validation by real-time quantitative PCR (qPCR). qPCR was also used to examine the expression of additional genes known to be important in the adaptive immune response.
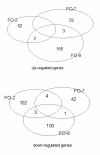
Results: Granulomatous lesions were observed in all vaccinated fish. The presence of severe granulomas was associated with a profile of up-regulation of innate immunity-related genes such as complement factors C1q and C6, mannose binding protein, lysozyme C, C-type lectin receptor, CD209, Cathepsin D, CD63, LECT-2, CC chemokine and metallothionein. In addition, TGF-beta (p = 0.001), IL-17A (p = 0.007) and its receptor (IL-17AR) (p = 0.009) representing TH17 were significantly up-regulated in the group with severe granulomas as were arginase and IgM. None of the genes directly reflective of T(H)1 T cell lineage (IFN-gamma, CD4) or T(H)2 (GATA-3) responses were differentially expressed.
Conclusions: Granulomatous reactions following vaccination with oil-based vaccines in Atlantic salmon have the profile of strong expression of genes related to innate immune responses. The expression of TGF-beta, IL-17A and its receptor suggests an involvement of T(H)17 T cell lineage and is in conformity with strong infiltration of neutrophils and macrophages into inflamed areas. Arginase upregulation shows that macrophages in these reactions are alternatively activated, indicating also a T(H)2-profile. To what extent the expression of IL-17A and its receptor reflects an autoimmune vaccine-based reaction remains elusive but would be in conformity with previous observations of autoimmune reactions in salmon when vaccinated with oil-based vaccines.
Figures




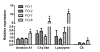
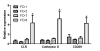
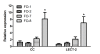



Similar articles
-
The contribution of Aeromonas salmonicida extracellular products to the induction of inflammation in Atlantic salmon (Salmo salar L.) following vaccination with oil-based vaccines.Fish Shellfish Immunol. 2006 Jan;20(1):1-11. doi: 10.1016/j.fsi.2005.01.005. Fish Shellfish Immunol. 2006. PMID: 16018934
-
Transcriptomic Profiling of the Adaptive and Innate Immune Responses of Atlantic Salmon to Renibacterium salmoninarum Infection.Front Immunol. 2020 Oct 28;11:567838. doi: 10.3389/fimmu.2020.567838. eCollection 2020. Front Immunol. 2020. PMID: 33193341 Free PMC article.
-
Vaccination-induced systemic autoimmunity in farmed Atlantic salmon.J Immunol. 2008 Oct 1;181(7):4807-14. doi: 10.4049/jimmunol.181.7.4807. J Immunol. 2008. PMID: 18802084
-
Vaccinated fish welfare: protection versus side-effects.Dev Biol Stand. 1997;90:371-9. Dev Biol Stand. 1997. PMID: 9270866 Review.
-
T cell-macrophage interactions and granuloma formation in vasculitis.Front Immunol. 2014 Sep 12;5:432. doi: 10.3389/fimmu.2014.00432. eCollection 2014. Front Immunol. 2014. PMID: 25309534 Free PMC article. Review.
Cited by
-
Transcription Factor T-Bet in Atlantic Salmon: Characterization and Gene Expression in Mucosal Tissues during Aeromonas Salmonicida Infection.Front Immunol. 2015 Jul 6;6:345. doi: 10.3389/fimmu.2015.00345. eCollection 2015. Front Immunol. 2015. PMID: 26217339 Free PMC article.
-
Dynamics of Polarized Macrophages and Activated CD8+ Cells in Heart Tissue of Atlantic Salmon Infected With Piscine Orthoreovirus-1.Front Immunol. 2021 Sep 16;12:729017. doi: 10.3389/fimmu.2021.729017. eCollection 2021. Front Immunol. 2021. PMID: 34603301 Free PMC article.
-
Advances in Vaccine Adjuvants for Teleost Fish: Implications for Aquatic Welfare and the Potential of Nanoparticle-Based Formulations.Vaccines (Basel). 2024 Nov 28;12(12):1347. doi: 10.3390/vaccines12121347. Vaccines (Basel). 2024. PMID: 39772009 Free PMC article. Review.
-
Transforming Aquaculture through Vaccination: A Review on Recent Developments and Milestones.Vaccines (Basel). 2024 Jul 1;12(7):732. doi: 10.3390/vaccines12070732. Vaccines (Basel). 2024. PMID: 39066370 Free PMC article. Review.
-
Cellular Immune Responses in Rainbow Trout (Onchorhynchus mykiss) Following Vaccination and Challenge Against Salmonid Alphavirus (SAV).Vaccines (Basel). 2020 Dec 2;8(4):725. doi: 10.3390/vaccines8040725. Vaccines (Basel). 2020. PMID: 33276596 Free PMC article.
References
-
- Evensen Ø, Brudeseth B, Mutoloki S. The vaccine formulation and its role in inflammatory processes in fish - effects and adverse effects. Dev Biol (Basel) 2005;121:117–125. - PubMed
-
- Mutoloki S, Alexandersen S, Evensen Ø. Sequential study of antigen persistence and concomitant inflammatory reactions relative to side effects and growth of Atlantic salmon (Salmo salar L.) following intraperitoneal injection with oil adjuvanted vaccines. Fish Shellfish Immunol. 2004;16:633–645. doi: 10.1016/j.fsi.2003.10.002. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

