Arecoline induces HA22T/VGH hepatoma cells to undergo anoikis - involvement of STAT3 and RhoA activation
- PMID: 20507639
- PMCID: PMC2895595
- DOI: 10.1186/1476-4598-9-126
Arecoline induces HA22T/VGH hepatoma cells to undergo anoikis - involvement of STAT3 and RhoA activation
Abstract
Background: Our previous study showed that, in basal cell carcinoma cells, arecoline reduces levels of the tumor cell survival factor interleukin-6 (IL-6), increases levels of tumor suppressor factor p53, and elicits cell cycle arrest, followed by apoptosis. In preliminarily studies, we observed that arecoline induces detachment of the human-derived hepatoma cell line HA22T/VGH from the extracellular matrix. In the present study, we explored the fate of the detached HA22T/VGH cells and investigated the underlying mechanism.
Methods: HA22T/VGH cells or primary cultured rat hepatocytes were treated with arecoline, then changes in morphology, viability, apoptosis, and the expression of surface beta1-integrin, apoptosis-related proteins, and IL-6 were examined. Furthermore, activation of the signal transducer and activator of transcription 3 (STAT3) pathway and the RhoA/Rock signaling pathway, including p190RhoGAP and Src homology-2 domain-containing phosphatase SHP2, was examined.
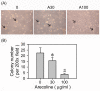
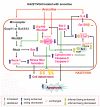
Results: A low concentration of arecoline (<or= 100 microg/ml) caused cytoskeletal changes in HA22T/VGH cells, but not hepatocytes, and this was accompanied by decreased beta1-integrin expression and followed by apoptosis, indicating that HA22T/VGH cells undergo anoikis after arecoline treatment. IL-6 expression and phosphorylation of STAT3, which provides protection against anoikis, were inhibited and levels of downstream signaling proteins, including Bcl-XL and Bcl-2, were decreased, while Bax expression, mitochondrial cytochrome c release, and caspase-3 activity were increased. In addition, phosphorylation/activation of p190RhoGAP, a RhoA inhibitor, and of its upstream regulator, SHP2, was inhibited by arecoline treatment, while Rho/Rock activation was increased. Addition of the RhoA inhibitor attenuated the effects of arecoline.
Conclusions: This study demonstrated that arecoline induces anoikis of HA22T/VGH cells involving inhibition of STAT3 and increased RhoA/Rock activation and that the STAT3 and RhoA/Rock signaling pathways are connected.
Figures



Similar articles
-
Arecoline Increases Glycolysis and Modulates pH Regulator Expression in HA22T/VGH Hepatoma Cells, Leading to Increase of Intracellular Ca2+, Reactive Oxygen Species, and Anoikis.J Cancer. 2017 Sep 15;8(16):3173-3182. doi: 10.7150/jca.20523. eCollection 2017. J Cancer. 2017. PMID: 29158789 Free PMC article.
-
Constitutive production of colony-stimulating factors by human hepatoma cell lines: possible correlation with cell differentiation.Exp Hematol. 1996 Feb;24(3):437-44. Exp Hematol. 1996. PMID: 8599973
-
Antitumor effects of the novel NF-kappaB inhibitor dehydroxymethyl-epoxyquinomicin on human hepatic cancer cells: analysis of synergy with cisplatin and of possible correlation with inhibition of pro-survival genes and IL-6 production.Int J Oncol. 2006 Apr;28(4):923-30. Int J Oncol. 2006. PMID: 16525642
-
Pharmacologically inducing anoikis offers novel therapeutic opportunities in hepatocellular carcinoma.Biomed Pharmacother. 2024 Jul;176:116878. doi: 10.1016/j.biopha.2024.116878. Epub 2024 Jun 5. Biomed Pharmacother. 2024. PMID: 38843588 Review.
-
Novel anti-cancer compounds for developing combinatorial therapies to target anoikis-resistant tumors.Pharm Res. 2012 Mar;29(3):621-36. doi: 10.1007/s11095-011-0645-9. Epub 2011 Dec 28. Pharm Res. 2012. PMID: 22203324 Review.
Cited by
-
Arecoline promotes proliferation and migration of human HepG2 cells through activation of the PI3K/AKT/mTOR pathway.Hereditas. 2022 Jul 14;159(1):29. doi: 10.1186/s41065-022-00241-0. Hereditas. 2022. PMID: 35836300 Free PMC article.
-
Involvement of MicroRNA-296 in the Inhibitory Effect of Epigallocatechin Gallate against the Migratory Properties of Anoikis-Resistant Nasopharyngeal Carcinoma Cells.Cancers (Basel). 2020 Apr 15;12(4):973. doi: 10.3390/cancers12040973. Cancers (Basel). 2020. PMID: 32326395 Free PMC article.
-
Zinc Protects Articular Chondrocytes through Changes in Nrf2-Mediated Antioxidants, Cytokines and Matrix Metalloproteinases.Nutrients. 2018 Apr 11;10(4):471. doi: 10.3390/nu10040471. Nutrients. 2018. PMID: 29641501 Free PMC article.
-
Induction of cell cycle arrest by increasing GTP‑RhoA levels via Taxol‑induced microtubule polymerization in renal cell carcinoma.Mol Med Rep. 2017 Jun;15(6):4273-4279. doi: 10.3892/mmr.2017.6543. Epub 2017 May 3. Mol Med Rep. 2017. PMID: 28487984 Free PMC article.
-
Vitamin C Protects Chondrocytes against Monosodium Iodoacetate-Induced Osteoarthritis by Multiple Pathways.Int J Mol Sci. 2016 Dec 27;18(1):38. doi: 10.3390/ijms18010038. Int J Mol Sci. 2016. PMID: 28035982 Free PMC article.
References
-
- Gray JA, Enz A, Spiegel R. Muscarinic agonists for senile dementia: past experience and future trends. Trends Pharmacol Sci. 1989. pp. 85–88. - PubMed
-
- Raffaele KC, Berardi A, Asthana S, Morris P, Haxby JV, Soncrant TT. Effects of long-term continuous infusion of the muscarinic cholinergic agonist arecoline on verbal memory in dementia of the Alzheimer type. Psychopharmacol Bull. 1991;27:315–319. - PubMed
-
- Chang MC, Wu HL, Lee JJ, Lee PH, Chang HH, Hahn LJ, Lin BR, Chen YJ, Jeng JH. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J Biol Chem. 2004;279:50676–50683. doi: 10.1074/jbc.M404465200. - DOI - PubMed
-
- Jeng JH, Wang YJ, Chiang BL, Lee PH, Chan CP, Ho YS, Wang TM, Lee JJ, Hahn LJ, Chang MC. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;24:1301–1315. doi: 10.1093/carcin/bgg083. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

