Calcium sensitivity and the Frank-Starling mechanism of the heart are increased in titin N2B region-deficient mice
- PMID: 20507834
- PMCID: PMC2917497
- DOI: 10.1016/j.yjmcc.2010.05.006
Calcium sensitivity and the Frank-Starling mechanism of the heart are increased in titin N2B region-deficient mice
Abstract
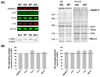
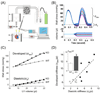
Previous work suggests that titin-based passive tension is a factor in the Frank-Starling mechanism of the heart, by increasing length-dependent activation (LDA) through an increase in calcium sensitivity at long sarcomere length. We tested this hypothesis in a mouse model (N2B KO model) in which titin-based passive tension is elevated as a result of the excision of the N2B element, one of cardiac titin's spring elements. LDA was assessed by measuring the active tension-pCa (-log[Ca(2+)]) relationship at sarcomere length (SLs) of 1.95, 2.10, and 2.30 microm in WT and N2B KO skinned myocardium. LDA was positively correlated with titin-based passive tension due to an increase in calcium sensitivity at the longer SLs in the KO. For example, at pCa 6.0, the KO:WT tension ratio was 1.28+/-0.07 and 1.42+/-0.04 at SLs of 2.1 and 2.3 microm, respectively. There was no difference in protein expression or total phosphorylation of sarcomeric proteins. We also measured the calcium sensitivity after PKA treating the skinned muscle and found that titin-based passive tension was also now correlated with LDA, with a slope that was significantly increased compared to no PKA treatment. Finally, we performed isolated heart experiments and measured the Frank-Starling relation (slope of developed wall stress-LV volume relation) as well as diastolic stiffness (slope of diastolic wall stress-volume relation). The FSM was more pronounced in the N2B KO hearts and the slope of the FSM correlated with diastolic stiffness. These findings support that titin-based passive tension triggers an increase in calcium sensitivity at long sarcomere length, thereby playing an important role in the Frank-Starling mechanism of the heart.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Calcium sensitivity and myofilament lattice structure in titin N2B KO mice.Arch Biochem Biophys. 2013 Jul 1;535(1):76-83. doi: 10.1016/j.abb.2012.12.004. Epub 2012 Dec 14. Arch Biochem Biophys. 2013. PMID: 23246787 Free PMC article.
-
Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3444-9. doi: 10.1073/pnas.0608543104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360664 Free PMC article.
-
Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart.Circulation. 2001 Oct 2;104(14):1639-45. doi: 10.1161/hc3901.095898. Circulation. 2001. PMID: 11581142
-
Titin/connectin-based modulation of the Frank-Starling mechanism of the heart.J Muscle Res Cell Motil. 2005;26(6-8):319-23. doi: 10.1007/s10974-005-9038-1. J Muscle Res Cell Motil. 2005. PMID: 16453158 Review.
-
Titin: an endosarcomeric protein that modulates myocardial stiffness in DCM.J Card Fail. 2002 Dec;8(6 Suppl):S276-86. doi: 10.1054/jcaf.2002.129278. J Card Fail. 2002. PMID: 12555133 Review.
Cited by
-
The titin N2B and N2A regions: biomechanical and metabolic signaling hubs in cross-striated muscles.Biophys Rev. 2021 Sep 9;13(5):653-677. doi: 10.1007/s12551-021-00836-3. eCollection 2021 Oct. Biophys Rev. 2021. PMID: 34745373 Free PMC article. Review.
-
Titin (TTN): from molecule to modifications, mechanics, and medical significance.Cardiovasc Res. 2022 Nov 10;118(14):2903-2918. doi: 10.1093/cvr/cvab328. Cardiovasc Res. 2022. PMID: 34662387 Free PMC article. Review.
-
CaMKII effects on inotropic but not lusitropic force frequency responses require phospholamban.J Mol Cell Cardiol. 2012 Sep;53(3):429-36. doi: 10.1016/j.yjmcc.2012.06.019. Epub 2012 Jul 11. J Mol Cell Cardiol. 2012. PMID: 22796260 Free PMC article.
-
Stretch of contracting cardiac muscle abruptly decreases the rate of phosphate release at high and low calcium.J Biol Chem. 2012 Jul 27;287(31):25696-705. doi: 10.1074/jbc.M112.373498. Epub 2012 Jun 12. J Biol Chem. 2012. PMID: 22692210 Free PMC article.
-
Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues.Adv Drug Deliv Rev. 2016 Jan 15;96:110-34. doi: 10.1016/j.addr.2015.04.019. Epub 2015 May 5. Adv Drug Deliv Rev. 2016. PMID: 25956564 Free PMC article. Review.
References
-
- Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Journal. 1998;83:471–480. - PubMed
-
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. Journal. 1985;17:821–840. - PubMed
-
- Shiels HA, White E. The Frank-Starling mechanism in vertebrate cardiac myocytes. Journal. 2008;211:2005–2013. - PubMed
-
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Journal. 1986;58:755–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

