Human L-selectin preferentially binds synthetic glycosulfopeptides modeled after endoglycan and containing tyrosine sulfate residues and sialyl Lewis x in core 2 O-glycans
- PMID: 20507883
- PMCID: PMC2948818
- DOI: 10.1093/glycob/cwq083
Human L-selectin preferentially binds synthetic glycosulfopeptides modeled after endoglycan and containing tyrosine sulfate residues and sialyl Lewis x in core 2 O-glycans
Abstract
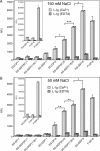
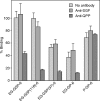
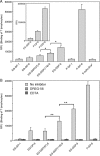
Endoglycan is a mucin-like glycoprotein expressed by endothelial cells and some leukocytes and is recognized by L-selectin, a C-type lectin important in leukocyte trafficking and extravasation during inflammation. Here, we show that recombinant L-selectin and human T lymphocytes expressing L-selectin bind to synthetic glycosulfopeptides (GSPs). These synthetic glycosulfopeptides contain 37 amino acid residues modeled after the N-terminus of human endoglycan and contain one or two tyrosine sulfates (TyrSO(3)) along with a nearby core-2-based Thr-linked O-glycan with sialyl Lewis x (C2-SLe(x)). TyrSO(3) at position Y118 was more critical for binding than at Y97. C2-SLe(x) at T124 was required for L-selectin recognition. Interestingly, under similar conditions, neither L-selectin nor T lymphocytes showed appreciable binding to the sulfated carbohydrate epitope 6-sulfo-SLe(x). P-selectin also bound to endoglycan-based GSPs but with lower affinity than toward GSPs modeled after PSGL-1, the physiological ligand for P- and L-selectin that is expressed on leukocytes. These results demonstrate that TyrSO(3) residues in association with a C2-SLe(x) moiety within endoglycan and PSGL-1 are preferentially recognized by L-selectin.
Figures





References
-
- Baumhueter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. - PubMed
-
- Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–698. - PubMed
-
- Bernimoulin MP, Zeng XL, Abbal C, Giraud S, Martinez M, Michielin O, Schapira M, Spertini O. Molecular basis of leukocyte rolling on PSGL-1. Predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. J Biol Chem. 2003;278:37–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

