Specification of the NF-kappaB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKK epsilon
- PMID: 20507904
- PMCID: PMC2952868
- DOI: 10.1093/nar/gkq439
Specification of the NF-kappaB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKK epsilon
Abstract
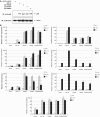
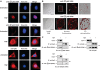
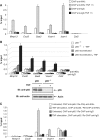
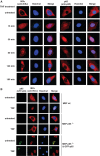
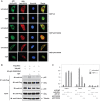
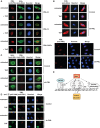
Here we investigated the regulation of NF-κB activity by post-translational modifications upon reconstitution of NF-κB p65-deficient cells with the wild-type protein or phosphorylation-defect mutants. Analysis of NF-κB target gene expression showed that p65 phosphorylations alone or in combination function to direct transcription in a highly target gene-specific fashion, a finding discussed here as the NF-κB barcode hypothesis. High-resolution microscopy and surface rendering revealed serine 536 phosphorylated p65 predominantly in the cytosol, while serine 468 phosphorylated p65 mainly localized in nuclear speckles. TNF stimulation resulted in the translocation of the cytosolic p65 kinase IKKε to the nucleus and also to promyelocytic leukemia (PML) nuclear bodies. This inducible IKKε translocation was dependent on p65 phosphorylation and was prevented by the oncogenic PML-RARα fusion protein. Chromatin immunoprecipitation experiments revealed the inducible association of IKKε to the control regions of several NF-κB target genes. In the nucleus, the kinase contributes to the expression of a subset of NF-κB-regulated genes, thus revealing a novel role of IKKε for the control of nuclear NF-κB activity.
Figures


Similar articles
-
Inducible phosphorylation of NF-kappa B p65 at serine 468 by T cell costimulation is mediated by IKK epsilon.J Biol Chem. 2006 Mar 10;281(10):6175-83. doi: 10.1074/jbc.M508045200. Epub 2006 Jan 3. J Biol Chem. 2006. PMID: 16407239
-
Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway.J Biol Chem. 2005 Oct 14;280(41):34538-47. doi: 10.1074/jbc.M504943200. Epub 2005 Aug 16. J Biol Chem. 2005. PMID: 16105840
-
Inducible gene expression of IκB-kinase ε is dependent on nuclear factor-κB in human pulmonary epithelial cells.Biochem J. 2024 Jul 17;481(14):959-980. doi: 10.1042/BCJ20230461. Biochem J. 2024. PMID: 38941070
-
Post-translational modifications of p65: state of the art.Front Cell Dev Biol. 2024 Jul 10;12:1417502. doi: 10.3389/fcell.2024.1417502. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39050887 Free PMC article. Review.
-
Role of IKKε in the Metabolic Diseases: Physiology, Pathophysiology, and Pharmacology.Front Pharmacol. 2022 May 19;13:888588. doi: 10.3389/fphar.2022.888588. eCollection 2022. Front Pharmacol. 2022. PMID: 35662709 Free PMC article. Review.
Cited by
-
Regulation of TAK1/TAB1-mediated IL-1β signaling by cytoplasmic PPARβ/δ.PLoS One. 2013 Apr 30;8(4):e63011. doi: 10.1371/journal.pone.0063011. Print 2013. PLoS One. 2013. PMID: 23646170 Free PMC article.
-
The Influenza A Virus Genotype Determines the Antiviral Function of NF-κB.J Virol. 2016 Aug 12;90(17):7980-90. doi: 10.1128/JVI.00946-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27356900 Free PMC article.
-
Dietary Silk Peptide Inhibits LPS-Induced Inflammatory Responses by Modulating Toll-Like Receptor 4 (TLR4) Signaling.Biomolecules. 2020 May 15;10(5):771. doi: 10.3390/biom10050771. Biomolecules. 2020. PMID: 32429220 Free PMC article.
-
TRAF6 Phosphorylation Prevents Its Autophagic Degradation and Re-Shapes LPS-Triggered Signaling Networks.Cancers (Basel). 2021 Jul 19;13(14):3618. doi: 10.3390/cancers13143618. Cancers (Basel). 2021. PMID: 34298830 Free PMC article.
-
Dynamic phosphorylation of RelA on Ser42 and Ser45 in response to TNFα stimulation regulates DNA binding and transcription.Open Biol. 2016 Jul;6(7):160055. doi: 10.1098/rsob.160055. Open Biol. 2016. PMID: 27466442 Free PMC article.
References
-
- Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009;8:480–499. - PubMed
-
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. - PubMed
-
- Natoli G, De Santa F. Shaping alternative NF-kappaB-dependent gene expression programs: new clues to specificity. Cell Death Differ. 2006;13:693–696. - PubMed
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. - PubMed
-
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006;2006:re13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

