Augmentation of radiation response by motesanib, a multikinase inhibitor that targets vascular endothelial growth factor receptors
- PMID: 20507929
- PMCID: PMC3216115
- DOI: 10.1158/1078-0432.CCR-09-3385
Augmentation of radiation response by motesanib, a multikinase inhibitor that targets vascular endothelial growth factor receptors
Abstract
Background: Motesanib is a potent inhibitor of vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3, platelet-derived growth factor receptor, and Kit receptors. In this report we examine the interaction between motesanib and radiation in vitro and in head and neck squamous cell carcinoma (HNSCC) xenograft models.
Experimental design: In vitro assays were done to assess the impact of motesanib on VEGFR2 signaling pathways in human umbilical vein endothelial cells (HUVEC). HNSCC lines grown as tumor xenografts in athymic nude mice were utilized to assess the in vivo activity of motesanib alone and in combination with radiation.
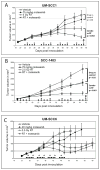
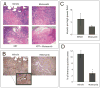
Results: Motesanib inhibited VEGF-stimulated HUVEC proliferation in vitro, as well as VEGFR2 kinase activity. Additionally, motesanib and fractionated radiation showed additive inhibitory effects on HUVEC proliferation. In vivo combination therapy with motesanib and radiation showed increased response compared with drug or radiation alone in UM-SCC1 (P < 0.002) and SCC-1483 xenografts (P = 0.001); however, the combination was not significantly more efficacious than radiation alone in UM-SCC6 xenografts. Xenografts treated with motesanib showed a reduction of vessel penetration into tumor parenchyma, compared with control tumors. Furthermore, triple immunohistochemical staining for vasculature, proliferation, and hypoxia showed well-defined spatial relationships among these parameters in HNSCC xenografts. Motesanib significantly enhanced intratumoral hypoxia in the presence and absence of fractionated radiation.
Conclusions: These studies identify a favorable interaction when combining radiation and motesanib in HNSCC models. The data presented suggest that motesanib reduces blood vessel penetration into tumors and thereby increases intratumoral hypoxia. These findings suggest that clinical investigations examining combinations of radiation and motesanib are warranted in HNSCC.
Copyright 2010 AACR.
Conflict of interest statement
Conflict of Interest Notification: AC and AP are employees of Amgen, Inc.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Pignon JP, le Maitre A, Bourhis J. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys. 2007;69:S112–4. - PubMed
-
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. - PubMed
-
- Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624–30. - PubMed
-
- Oc P, Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer. 2001;92:556–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

