Tombusvirus recruitment of host translational machinery via the 3' UTR
- PMID: 20507975
- PMCID: PMC2885689
- DOI: 10.1261/rna.2135210
Tombusvirus recruitment of host translational machinery via the 3' UTR
Abstract
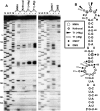
RNA viruses recruit the host translational machinery by different mechanisms that depend partly on the structure of their genomes. In this regard, the plus-strand RNA genomes of several different pathogenic plant viruses do not contain traditional translation-stimulating elements, i.e., a 5'-cap structure and a 3'-poly(A) tail, and instead rely on a 3'-cap-independent translational enhancer (3'CITE) located in their 3' untranslated regions (UTRs) for efficient synthesis of viral proteins. We investigated the structure and function of the I-shaped class of 3'CITE in tombusviruses--also present in aureusviruses and carmoviruses--using biochemical and molecular approaches and we determined that it adopts a complex higher-order RNA structure that facilitates translation by binding simultaneously to both eukaryotic initiation factor (eIF) 4F and the 5' UTR of the viral genome. The specificity of 3'CITE binding to eIF4F is mediated, at least in part, through a direct interaction with its eIF4E subunit, whereas its association with the viral 5' UTR relies on complementary RNA-RNA base-pairing. We show for the first time that this tripartite 5' UTR/3'CITE/eIF4F complex forms in vitro in a translationally relevant environment and is required for recruitment of ribosomes to the 5' end of the viral RNA genome by a mechanism that shares some fundamental features with cap-dependent translation. Notably, our results demonstrate that the 3'CITE facilitates the initiation step of translation and validate a molecular model that has been proposed to explain how several different classes of 3'CITE function. Moreover, the virus-host interplay defined in this study provides insights into natural host resistance mechanisms that have been linked to 3'CITE activity.
Figures







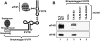




References
-
- Batten JS, Desvoyes B, Yamamura Y, Scholthof KB 2006. A translational enhancer element on the 3′-proximal end of the Panicum mosaic virus genome. FEBS Lett 580: 2591–2597 - PubMed
-
- Belsham GJ 2009. Divergent picornavirus IRES elements. Virus Res 139: 183–192 - PubMed
-
- Browning KS 1996. The plant translational apparatus. Plant Mol Biol 32: 107–144 - PubMed
-
- Browning KS, Humphreys J, Hobbs W, Smith GB, Ravel JM 1990. Determination of the amounts of the protein synthesis initiation and elongation factors in wheat germ. J Biol Chem 265: 17967–17973 - PubMed
-
- Browning KS, Webster C, Roberts JK, Ravel JM 1992. Identification of an isozyme form of protein synthesis initiation factor 4F in plants. J Biol Chem 267: 10096–10100 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
