Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency?
- PMID: 20507982
- PMCID: PMC2915671
- DOI: 10.1074/jbc.M110.115964
Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency?
Abstract
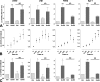
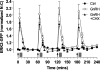
Gonadotropin-releasing hormone (GnRH) acts via G-protein-coupled receptors on gonadotrophs to stimulate synthesis and secretion of luteinizing hormone and follicle-stimulating hormone. It is secreted in pulses, and its effects depend on pulse frequency, but decoding mechanisms are unknown. Here we have used an extracellular signal regulated kinase-green fluorescent protein (ERK2-GFP) reporter to monitor GnRH signaling. GnRH caused dose-dependent ERK2-GFP translocation to the nucleus, providing a live-cell readout for activation. Pulsatile GnRH caused dose- and frequency-dependent ERK2-GFP translocation. These responses were rapid and transient, showed only digital tracking, and did not desensitize under any condition tested (dose, frequency, and receptor number varied). We also tested for the effects of cycloheximide (to prevent induction of nuclear-inducible MAPK phosphatases) and used GFP fusions containing ERK mutations (D319N, which prevents docking domain-dependent binding to MAPK phosphatases, and K52R, which prevents catalytic activity). These manipulations had little or no effect on the translocation responses, arguing against a role for MAPK phosphatases or ERK-mediated feedback in shaping ERK activation during pulsatile stimulation. GnRH also caused dose- and frequency-dependent activation of the alpha-gonadotropin subunit-, luteinizing hormone beta-, and follicle-stimulating hormone beta- luciferase reporters, and the latter response was inhibited by ERK1/2 knockdown. Moreover, GnRH caused frequency-dependent activation of an Egr1-luciferase reporter, but the response was proportional to cumulative pulse duration. Our data suggest that frequency decoding is not due to negative feedback shaping ERK signaling in this model.
Figures

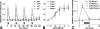
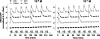
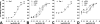
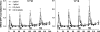
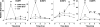

References
-
- Millar R. P., Lu Z. L., Pawson A. J., Flanagan C. A., Morgan K., Maudsley S. R. (2004) Endocr. Rev. 25, 235–275 - PubMed
-
- Burger L. L., Haisenleder D. J., Dalkin A. C., Marshall J. C. (2004) J. Mol. Endocrinol. 33, 559–584 - PubMed
-
- Naor Z. (2009) Front. Neuroendocrinol. 30, 10–29 - PubMed
-
- Ferris H. A., Shupnik M. A. (2006) Biol. Reprod. 74, 993–998 - PubMed
-
- Belchetz P. E., Plant T. M., Nakai Y., Keogh E. J., Knobil E. (1978) Science 202, 631–633 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

