Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile
- PMID: 20507987
- PMCID: PMC2915720
- DOI: 10.1074/jbc.M110.128546
Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile
Abstract
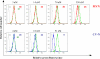
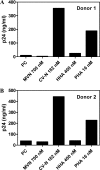
Microvirin (MVN), a recently isolated lectin from the cyanobacterium Microcystis aeruginosa PCC7806, shares 33% identity with the potent anti-human immunodeficiency virus (HIV) protein cyanovirin-N (CV-N) isolated from Nostoc ellipsosporum, and both lectins bind to similar carbohydrate structures. MVN is able to inhibit infection by a wide variety of HIV-1 laboratory-adapted strains and clinical isolates of different tropisms and subtypes in peripheral blood mononuclear cells. MVN also inhibits syncytium formation between persistently HIV-1-infected T cells and uninfected CD4(+) T cells and inhibits DC-SIGN-mediated HIV-1 binding and transmission to CD4(+) T cells. Long term passaging of HIV-1 exposed to dose-escalating concentrations of MVN resulted in the selection of a mutant virus with four deleted high mannose-type glycans in the envelope gp120. The MVN-resistant virus was still highly sensitive to various other carbohydrate binding lectins (e.g. CV-N, HHA, GNA, and UDA) but not anymore to the carbohydrate-specific 2G12 monoclonal antibody. Importantly, MVN is more than 50-fold less cytotoxic than CV-N. Also in sharp contrast to CV-N, MVN did not increase the level of the activation markers CD25, CD69, and HLA-DR in CD4(+) T lymphocytes, and subsequently, MVN did not enhance viral replication in pretreated peripheral blood mononuclear cells. Therefore, MVN may qualify as a useful lectin for potential microbicidal use based on its broad and potent antiviral activity and virtual lack of any stimulatory properties and cellular toxicity.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

