Elastase-mediated activation of the severe acute respiratory syndrome coronavirus spike protein at discrete sites within the S2 domain
- PMID: 20507992
- PMCID: PMC2906266
- DOI: 10.1074/jbc.M110.103275
Elastase-mediated activation of the severe acute respiratory syndrome coronavirus spike protein at discrete sites within the S2 domain
Abstract
Proteolytic priming is a common method of controlling the activation of membrane fusion mediated by viral glycoproteins. The severe acute respiratory syndrome coronavirus spike protein (SARS-CoV S) can be primed by a variety of host cell proteases, with proteolytic cleavage occurring both as the S1/S2 boundary and adjacent to a fusion peptide in the S2 domain. Here, we studied the priming of SARS-CoV S by elastase and show an important role for residue Thr(795) in the S2 domain. A series of alanine mutants were generated in the vicinity of the S2 cleavage site, with the goal of examining elastase-mediated cleavage within S2. Both proteolytic cleavage and fusion activation were modulated by altering the cleavage site position. We propose a novel mechanism whereby SARS-CoV fusion protein function can be controlled by spatial regulation of the proteolytic priming site, with important implications for viral pathogenesis.
Figures
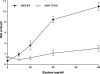
References
-
- Bosch B. J., Rottier P. J. (2008) in Nidoviruses (Perlman S., Gallagher T., Snijder E. J. eds) pp. 157–178, American Society for Microbiology Press, Washington, D. C.
-
- Klenk H.-D., Garten W. (1994) in Cellular Receptors for Animal Viruses (Wimmer E. ed) pp. 241–280, Cold Spring Harbor Press, Cold Spring Harbor, NY
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

