ppGpp conjures bacterial virulence
- PMID: 20508246
- PMCID: PMC2884408
- DOI: 10.1128/MMBR.00046-09
ppGpp conjures bacterial virulence
Abstract
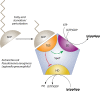
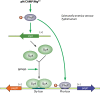
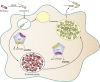
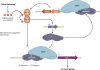
Like for all microbes, the goal of every pathogen is to survive and replicate. However, to overcome the formidable defenses of their hosts, pathogens are also endowed with traits commonly associated with virulence, such as surface attachment, cell or tissue invasion, and transmission. Numerous pathogens couple their specific virulence pathways with more general adaptations, like stress resistance, by integrating dedicated regulators with global signaling networks. In particular, many of nature's most dreaded bacteria rely on nucleotide alarmones to cue metabolic disturbances and coordinate survival and virulence programs. Here we discuss how components of the stringent response contribute to the virulence of a wide variety of pathogenic bacteria.
Figures



Similar articles
-
The stringent response and physiological roles of (pp)pGpp in bacteria.Nat Rev Microbiol. 2021 Apr;19(4):256-271. doi: 10.1038/s41579-020-00470-y. Epub 2020 Nov 4. Nat Rev Microbiol. 2021. PMID: 33149273 Review.
-
SpoT Induces Intracellular Salmonella Virulence Programs in the Phagosome.mBio. 2020 Feb 25;11(1):e03397-19. doi: 10.1128/mBio.03397-19. mBio. 2020. PMID: 32098823 Free PMC article.
-
(p)ppGpp: still magical?Annu Rev Microbiol. 2008;62:35-51. doi: 10.1146/annurev.micro.62.081307.162903. Annu Rev Microbiol. 2008. PMID: 18454629 Review.
-
ppGpp signaling plays a critical role in virulence of Acinetobacter baumannii.Virulence. 2021 Dec;12(1):2122-2132. doi: 10.1080/21505594.2021.1961660. Virulence. 2021. PMID: 34375563 Free PMC article.
-
Whole genome transcriptomics reveals global effects including up-regulation of Francisella pathogenicity island gene expression during active stringent response in the highly virulent Francisella tularensis subsp. tularensis SCHU S4.Microbiology (Reading). 2017 Nov;163(11):1664-1679. doi: 10.1099/mic.0.000550. Epub 2017 Oct 16. Microbiology (Reading). 2017. PMID: 29034854 Free PMC article.
Cited by
-
Ribosome•RelA structures reveal the mechanism of stringent response activation.Elife. 2016 Jul 19;5:e17029. doi: 10.7554/eLife.17029. Elife. 2016. PMID: 27434674 Free PMC article.
-
Global analysis of the Staphylococcus aureus response to mupirocin.Antimicrob Agents Chemother. 2012 Feb;56(2):787-804. doi: 10.1128/AAC.05363-11. Epub 2011 Nov 21. Antimicrob Agents Chemother. 2012. PMID: 22106209 Free PMC article.
-
Chemical inhibitors of the conserved bacterial transcriptional regulator DksA1 suppressed quorum sensing-mediated virulence of Pseudomonas aeruginosa.J Biol Chem. 2021 Jan-Jun;296:100576. doi: 10.1016/j.jbc.2021.100576. Epub 2021 Mar 21. J Biol Chem. 2021. PMID: 33757766 Free PMC article.
-
Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis.J Bacteriol. 2015 Apr;197(7):1146-56. doi: 10.1128/JB.02577-14. Epub 2015 Jan 20. J Bacteriol. 2015. PMID: 25605304 Free PMC article. Review.
-
Cationic bactericidal peptide 1018 does not specifically target the stringent response alarmone (p)ppGpp.Sci Rep. 2016 Nov 7;6:36549. doi: 10.1038/srep36549. Sci Rep. 2016. PMID: 27819280 Free PMC article.
References
-
- Aberg, A., V. Shingler, and C. Balsalobre. 2006. (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol. Microbiol. 60:1520-1533. - PubMed
-
- Aberg, A., V. Shingler, and C. Balsalobre. 2008. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol. Microbiol. 67:1223-1241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

