Gliding motility revisited: how do the myxobacteria move without flagella?
- PMID: 20508248
- PMCID: PMC2884410
- DOI: 10.1128/MMBR.00043-09
Gliding motility revisited: how do the myxobacteria move without flagella?
Abstract
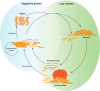
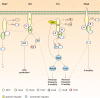
In bacteria, motility is important for a wide variety of biological functions such as virulence, fruiting body formation, and biofilm formation. While most bacteria move by using specialized appendages, usually external or periplasmic flagella, some bacteria use other mechanisms for their movements that are less well characterized. These mechanisms do not always exhibit obvious motility structures. Myxococcus xanthus is a motile bacterium that does not produce flagella but glides slowly over solid surfaces. How M. xanthus moves has remained a puzzle that has challenged microbiologists for over 50 years. Fortunately, recent advances in the analysis of motility mutants, bioinformatics, and protein localization have revealed likely mechanisms for the two M. xanthus motility systems. These results are summarized in this review.
Figures






Similar articles
-
Uncovering the mystery of gliding motility in the myxobacteria.Annu Rev Genet. 2011;45:21-39. doi: 10.1146/annurev-genet-110410-132547. Epub 2011 Sep 9. Annu Rev Genet. 2011. PMID: 21910630 Free PMC article. Review.
-
The mysterious nature of bacterial surface (gliding) motility: A focal adhesion-based mechanism in Myxococcus xanthus.Semin Cell Dev Biol. 2015 Oct;46:143-54. doi: 10.1016/j.semcdb.2015.10.033. Epub 2015 Oct 28. Semin Cell Dev Biol. 2015. PMID: 26520023 Review.
-
Gliding motility in bacteria: insights from studies of Myxococcus xanthus.Microbiol Mol Biol Rev. 1999 Sep;63(3):621-41. doi: 10.1128/MMBR.63.3.621-641.1999. Microbiol Mol Biol Rev. 1999. PMID: 10477310 Free PMC article. Review.
-
The elusive engine in Myxococcus xanthus gliding motility.Cell Mol Life Sci. 2007 Nov;64(21):2733-45. doi: 10.1007/s00018-007-7176-x. Cell Mol Life Sci. 2007. PMID: 17653507 Free PMC article. Review.
-
Flagella stator homologs function as motors for myxobacterial gliding motility by moving in helical trajectories.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):E1508-13. doi: 10.1073/pnas.1219982110. Epub 2013 Apr 1. Proc Natl Acad Sci U S A. 2013. PMID: 23576734 Free PMC article.
Cited by
-
First genomic insights into members of a candidate bacterial phylum responsible for wastewater bulking.PeerJ. 2015 Jan 27;3:e740. doi: 10.7717/peerj.740. eCollection 2015. PeerJ. 2015. PMID: 25650158 Free PMC article.
-
Conserved and diversified gene families of monovalent cation/h(+) antiporters from algae to flowering plants.Front Plant Sci. 2012 Feb 14;3:25. doi: 10.3389/fpls.2012.00025. eCollection 2012. Front Plant Sci. 2012. PMID: 22639643 Free PMC article.
-
Diversity of Myxobacteria-We Only See the Tip of the Iceberg.Microorganisms. 2018 Aug 11;6(3):84. doi: 10.3390/microorganisms6030084. Microorganisms. 2018. PMID: 30103481 Free PMC article. Review.
-
Data-driven modeling reveals cell behaviors controlling self-organization during Myxococcus xanthus development.Proc Natl Acad Sci U S A. 2017 Jun 6;114(23):E4592-E4601. doi: 10.1073/pnas.1620981114. Epub 2017 May 22. Proc Natl Acad Sci U S A. 2017. PMID: 28533367 Free PMC article.
-
Mutants defective in the production of encapsulin show a tan-phase-locked phenotype in Myxococcus xanthus.J Microbiol. 2019 Sep;57(9):795-802. doi: 10.1007/s12275-019-8683-9. Epub 2019 Jun 11. J Microbiol. 2019. PMID: 31187417
References
-
- Astling, D. P., J. Y. Lee, and D. R. Zusman. 2006. Differential effects of chemoreceptor methylation-domain mutations on swarming and development in the social bacterium Myxococcus xanthus. Mol. Microbiol. 59:45-55. - PubMed
-
- Bassler, B. L., and R. Losick. 2006. Bacterially speaking. Cell 125:237-246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

