The evolution of sex: a perspective from the fungal kingdom
- PMID: 20508251
- PMCID: PMC2884414
- DOI: 10.1128/MMBR.00005-10
The evolution of sex: a perspective from the fungal kingdom
Abstract
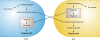
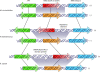
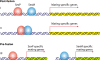
Sex is shrouded in mystery. Not only does it preferentially occur in the dark for both fungi and many animals, but evolutionary biologists continue to debate its benefits given costs in light of its pervasive nature. Experimental studies of the benefits and costs of sexual reproduction with fungi as model systems have begun to provide evidence that the balance between sexual and asexual reproduction shifts in response to selective pressures. Given their unique evolutionary history as opisthokonts, along with metazoans, fungi serve as exceptional models for the evolution of sex and sex-determining regions of the genome (the mating type locus) and for transitions that commonly occur between outcrossing/self-sterile and inbreeding/self-fertile modes of reproduction. We review here the state of the understanding of sex and its evolution in the fungal kingdom and also areas where the field has contributed and will continue to contribute to illuminating general principles and paradigms of sexual reproduction.
Figures

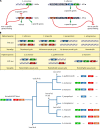
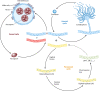

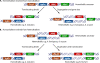


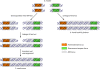

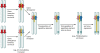



Similar articles
-
Evolution of fungal sexual reproduction.Mycologia. 2013 Jan-Feb;105(1):1-27. doi: 10.3852/12-253. Epub 2012 Oct 25. Mycologia. 2013. PMID: 23099518 Review.
-
Obligate sexual reproduction of a homothallic fungus closely related to the Cryptococcus pathogenic species complex.Elife. 2022 Jun 17;11:e79114. doi: 10.7554/eLife.79114. Elife. 2022. PMID: 35713948 Free PMC article.
-
Sex and the Imperfect Fungi.Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.funk-0043-2017. doi: 10.1128/microbiolspec.FUNK-0043-2017. Microbiol Spectr. 2017. PMID: 28597816 Free PMC article. Review.
-
Sex in the Mucoralean fungi.Mycoses. 2014 Dec;57 Suppl 3(0 3):18-24. doi: 10.1111/myc.12244. Epub 2014 Aug 31. Mycoses. 2014. PMID: 25175551 Free PMC article. Review.
-
Sex, outcrossing and mating types: unsolved questions in fungi and beyond.J Evol Biol. 2012 Jun;25(6):1020-38. doi: 10.1111/j.1420-9101.2012.02495.x. Epub 2012 Apr 20. J Evol Biol. 2012. PMID: 22515640 Review.
Cited by
-
Evolutionary erosion of yeast sex chromosomes by mating-type switching accidents.Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):20024-9. doi: 10.1073/pnas.1112808108. Epub 2011 Nov 28. Proc Natl Acad Sci U S A. 2011. PMID: 22123960 Free PMC article.
-
Sex-specific gene expression during asexual development of Neurospora crassa.Fungal Genet Biol. 2012 Jul;49(7):533-43. doi: 10.1016/j.fgb.2012.05.004. Epub 2012 May 22. Fungal Genet Biol. 2012. PMID: 22626843 Free PMC article.
-
More Filtering on SNP Calling Does Not Remove Evidence of Inter-Nucleus Recombination in Dikaryotic Arbuscular Mycorrhizal Fungi.Front Plant Sci. 2020 Jul 7;11:912. doi: 10.3389/fpls.2020.00912. eCollection 2020. Front Plant Sci. 2020. PMID: 32733503 Free PMC article.
-
Evolution of asexual and sexual reproduction in the aspergilli.Stud Mycol. 2018 Sep;91:37-59. doi: 10.1016/j.simyco.2018.10.002. Epub 2018 Oct 11. Stud Mycol. 2018. PMID: 30425416 Free PMC article.
-
Unveiling Cryptic Species Diversity and Genetic Variation of Lasiodiplodia (Botryosphaeriaceae, Botryosphaeriales) Infecting Fruit Crops in Taiwan.J Fungi (Basel). 2023 Sep 20;9(9):950. doi: 10.3390/jof9090950. J Fungi (Basel). 2023. PMID: 37755058 Free PMC article.
References
-
- Alexopoulos, C. J. 1962. Introductory mycology, 2nd ed. John Wiley & Sons, New York, NY.
-
- Alexopoulos, C. J., C. W. Mims, and M. Blackwell. 1996. Introductory mycology, 4th ed. John Wiley & Sons, New York, NY.
-
- Alvarez-Perez, S., J. L. Blanco, P. Alba, and M. E. Garcia. 2009. Mating type and invasiveness are significantly associated in Aspergillus fumigatus. Med. Mycol. 26:1-6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

