Tetramic and tetronic acids as scaffolds in bioinorganic and bioorganic chemistry
- PMID: 20508811
- PMCID: PMC2875697
- DOI: 10.1155/2010/315056
Tetramic and tetronic acids as scaffolds in bioinorganic and bioorganic chemistry
Abstract
Tetramic and tetronic acids are naturally occurring molecules with a variety of biological activities. In this review article, we present the general strategies for the synthesis of these compounds and we reveal the functionalized groups that are responsible for their properties. We also set out their coordinating modes with up-to-date bibliographical references.
Figures

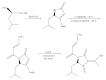
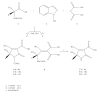
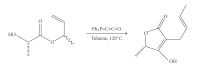
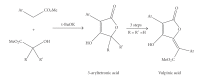
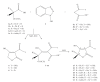
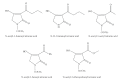
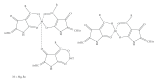
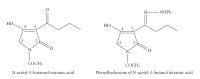
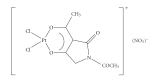
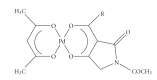
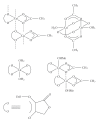
References
-
- Royles BJL. Naturally occurring tetramic acids: structure, isolation, and synthesis. 1995;95(6):1981–2001.
-
- Schobert R, Schlenk A. Tetramic and tetronic acids: an update on new derivatives and biological aspects. 2008;16(8):4203–4221. - PubMed
-
- Aoki S, Higuchi K, Ye Y, Satari R, Kobayashi M. Melophlins A and B, novel tetramic acids reversing the phenotype of ras- transformed cells, from the marine sponge Melophlus sarassinorum. 2000;56(13):1833–1836.
-
- Phillips NJ, Goodwin JT, Fraiman A, Cole RJ, Lynn DG. Characterization of the Fusarium toxin equisetin: the use of phenylboronates in structure assignment. 1989;111(21):8223–8231.
LinkOut - more resources
Full Text Sources

