Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review
- PMID: 20508843
- PMCID: PMC2874930
- DOI: 10.1155/2010/513948
Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review
Abstract
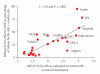
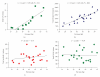
This paper considers the role of putative adipokines that might be involved in the enhanced inflammatory response of human adipose tissue seen in obesity. Inflammatory adipokines [IL-6, IL-10, ACE, TGFbeta1, TNFalpha, IL-1beta, PAI-1, and IL-8] plus one anti-inflammatory [IL-10] adipokine were identified whose circulating levels as well as in vitro release by fat are enhanced in obesity and are primarily released by the nonfat cells of human adipose tissue. In contrast, the circulating levels of leptin and FABP-4 are also enhanced in obesity and they are primarily released by fat cells of human adipose tissue. The relative expression of adipokines and other proteins in human omental as compared to subcutaneous adipose tissue as well as their expression in the nonfat as compared to the fat cells of human omental adipose tissue is also reviewed. The conclusion is that the release of many inflammatory adipokines by adipose tissue is enhanced in obese humans.
Figures



References
-
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. British Journal of Nutrition. 2004;92(3):347–355. - PubMed
-
- Engeli S, Sharma AM. Role of adipose tissue for cardiovascular-renal regulation in health and disease. Hormone and Metabolic Research. 2000;32(11-12):485–499. - PubMed
-
- Ferrante AW., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. Journal of Internal Medicine. 2007;262(4):408–414. - PubMed
-
- Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. - PubMed
-
- Clement K, Langin D. Regulation of inflammation-related genes in human adipose tissue. Journal of Internal Medicine. 2007;262(4):422–430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

