Assembly mechanism of the sixty-subunit nanoparticles via interaction of RNA with the reengineered protein connector of phi29 DNA-packaging motor
- PMID: 20509670
- PMCID: PMC2889630
- DOI: 10.1021/nn100158k
Assembly mechanism of the sixty-subunit nanoparticles via interaction of RNA with the reengineered protein connector of phi29 DNA-packaging motor
Abstract
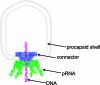
Bacterial virus phi29 genomic DNA is packaged into a procapsid shell with the aid of a motor containing a 12-subunit connector channel and a hexameric pRNA (packaging RNA) ring. The wide end, or the C-terminus, of the cone-shaped connector is embedded within the procapsid shell, whereas the narrow end, or N-terminus, extends outside of the procapsid, providing a binding location for pRNA. Recently, we have reported the mechanism of in vivo assembly of an ellipsoid nanoparticle with seven connectors through an interaction among a peptide tag. Here we report the formation of a similar nanoparticle in vitro via the addition of DNA or RNA oligos to connector proteins. Free connectors guided by one or two copies of oligonucleotides were assembled into a rosette structure containing 60 subunits of reengineered proteins. The number of oligonucleotides within the particle is length-dependent but sequence-independent. Reversible shifting between the 12- and 60-subunit nanoparticles (between individual connectors and rosette structures, respectively) was demonstrated by the alternative addition of oligonucleotides and the treatment of ribonuclease, suggesting a potential application as a switch or regulator in nanobiotechnology. This advancement allows for a simple method to produce multivalent nanoparticles that contain five 12-unit nanoparticles with defined structure and stoichiometry. That is, it will be possible to assemble nanoparticles in vitro with the combination of 60 assortments of ligands, tags, therapeutic drugs, and diagnostic moieties for multivalent delivery or enhancement of signal detection in nanotechnological and nanomedicinal applications.
Figures





Similar articles
-
Methods for Single-Molecule Sensing and Detection Using Bacteriophage Phi29 DNA Packaging Motor.Methods Mol Biol. 2018;1805:423-450. doi: 10.1007/978-1-4939-8556-2_21. Methods Mol Biol. 2018. PMID: 29971730
-
Grouping of ferritin and gold nanoparticles conjugated to pRNA of the phage phi29 DNA-packaging motor.J Nanosci Nanotechnol. 2007 Sep;7(9):3257-67. doi: 10.1166/jnn.2007.914. J Nanosci Nanotechnol. 2007. PMID: 18019159
-
Construction and 3-D computer modeling of connector arrays with tetragonal to decagonal transition induced by pRNA of phi29 DNA-packaging motor.J Nanosci Nanotechnol. 2005 Jun;5(6):856-63. doi: 10.1166/jnn.2005.143. J Nanosci Nanotechnol. 2005. PMID: 16060143
-
RNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapy.J Nanosci Nanotechnol. 2005 Dec;5(12):1964-82. doi: 10.1166/jnn.2005.446. J Nanosci Nanotechnol. 2005. PMID: 16430131 Free PMC article. Review.
-
Ultrastable pRNA hexameric ring gearing hexameric phi29 DNA-packaging motor by revolving without rotating and coiling.Curr Opin Biotechnol. 2013 Aug;24(4):581-90. doi: 10.1016/j.copbio.2013.03.019. Epub 2013 May 14. Curr Opin Biotechnol. 2013. PMID: 23683853 Free PMC article. Review.
Cited by
-
Revolution rather than rotation of AAA+ hexameric phi29 nanomotor for viral dsDNA packaging without coiling.Virology. 2013 Aug 15;443(1):28-39. doi: 10.1016/j.virol.2013.04.019. Epub 2013 Jun 12. Virology. 2013. PMID: 23763768 Free PMC article.
-
Mechanism of one-way traffic of hexameric phi29 DNA packaging motor with four electropositive relaying layers facilitating antiparallel revolution.ACS Nano. 2013 May 28;7(5):4082-92. doi: 10.1021/nn4002775. Epub 2013 Mar 26. ACS Nano. 2013. PMID: 23510192 Free PMC article.
-
Discovery of a new motion mechanism of biomotors similar to the earth revolving around the sun without rotation.Virology. 2013 Nov;446(1-2):133-43. doi: 10.1016/j.virol.2013.07.025. Epub 2013 Aug 27. Virology. 2013. PMID: 24074575 Free PMC article. Review.
-
Cryo-EM Structures of Two Bacteriophage Portal Proteins Provide Insights for Antimicrobial Phage Engineering.Viruses. 2021 Dec 16;13(12):2532. doi: 10.3390/v13122532. Viruses. 2021. PMID: 34960800 Free PMC article.
-
A boost for the emerging field of RNA nanotechnology.ACS Nano. 2011 May 24;5(5):3405-18. doi: 10.1021/nn200989r. ACS Nano. 2011. PMID: 21604810 Free PMC article.
References
-
- Heymann J. B.; Cheng N. Q.; Newcomb W. W.; Trus B. L.; Brown J. C.; Steven A. C. Dynamics of Herpes Simplex Virus Capsid Maturation Visualized by Time-Lapse Cryo-Electron Microscopy. Nat. Struct. Biol. 2003, 10, 334–341. - PubMed
-
- Cusack S. Adenovirus Complex Structures. Curr. Opin. Struct. Biol. 2005, 15, 237–243. - PubMed
-
- Catalano C. E.; Cue D.; Feiss M. Virus DNA Packaging: The Strategy Used by Phage Lambda. Mol. Microbiol. 1995, 16, 1075–1086. - PubMed
-
- Camacho A. G.; Gual A.; Lurz R.; Tavares P.; Alonso J. C. Bacillus subtilis Bacteriophage SPP1 DNA Packaging Motor Requires Terminase and Portal Proteins. J. Biol. Chem. 2003, 278, 23251–23259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

