Tumor cell behaviour modulation by mesenchymal stromal cells
- PMID: 20509882
- PMCID: PMC2890609
- DOI: 10.1186/1476-4598-9-129
Tumor cell behaviour modulation by mesenchymal stromal cells
Abstract
Background: Human mesenchymal stromal cells (MSC) hold a promise for future cell-based therapies due to their immunomodulatory properties and/or secretory activity. Nevertheless non-neoplastic tumor compartment could also originate from MSC. We aimed to show whether multipotent MSC derived from human adipose tissue (AT-MSC) could create tumor cell-protective milieu and affect tumor cell behaviour in vitro and in vivo.
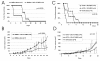
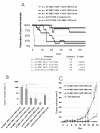
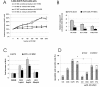
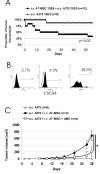
Results: Here we have demonstrated tumor-promoting effect of AT-MSC on human melanoma A375 cells. AT-MSC coinjection mediated abrogation of tumor latency and supported subcutaneous xenotransplant growth from very low melanoma cell doses. Tumor incidence was also significantly increased by AT-MSC-derived soluble factors. AT-MSC supported proliferation, suppressed apoptosis and modulated melanoma cell responses to cytotoxic drugs in vitro. Expression and multiplex cytokine assays confirmed synergistic increase in VEGF that contributed to the AT-MSC-mediated support of A375 xenotransplant growth. Production of G-CSF and other factors implicated in formation of supportive proinflammatory tumor cell microenvironment was also confirmed. SDF-1alpha/CXCR4 signalling contributed to tumor-promoting effect of systemic AT-MSC administration on A375 xenotransplants. However, no support was observed for human glioblastoma cells 8MGBA co-injected along with AT-MSC that did not sustain tumor xenotransplant growth in vivo. Tumor-inhibiting response could be attributed to the synergistic action of multiple cytokines produced by AT-MSC on glioblastoma cells.
Conclusions: Herein we provide experimental evidence for MSC-mediated protective effect on melanoma A375 cells under nutrient-limiting and hostile environmental conditions resulting from mutual crosstalk between neoplastic and non-malignant cells. This tumor-favouring effect was not observed for the glioblastoma cells 8MGBA. Collectively, our data further strengthen the need for unravelling mechanisms underlying MSC-mediated modulation of tumor behaviour for possible future MSC clinical use in the context of malignant disease.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

