Antiproliferative effect of growth hormone-releasing hormone (GHRH) antagonist on ovarian cancer cells through the EGFR-Akt pathway
- PMID: 20509930
- PMCID: PMC2891788
- DOI: 10.1186/1477-7827-8-54
Antiproliferative effect of growth hormone-releasing hormone (GHRH) antagonist on ovarian cancer cells through the EGFR-Akt pathway
Abstract
Background: Antagonists of growth hormone-releasing hormone (GHRH) are being developed for the treatment of various human cancers.
Methods: MTT assay was used to test the proliferation of SKOV3 and CaOV3. The splice variant expression of GHRH receptors was examined by RT-PCR. The expression of protein in signal pathway was examined by Western blotting. siRNA was used to block the effect of EGFR.
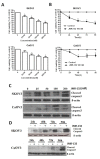
Results: In this study, we investigated the effects of a new GHRH antagonist JMR-132, in ovarian cancer cell lines SKOV3 and CaOV3 expressing splice variant (SV)1 of GHRH receptors. MTT assay showed that JMR-132 had strong antiproliferative effects on SKOV3 and CaOV3 cells in both a time-dependent and dose-dependent fashion. JMR-132 also induced the activation and increased cleaved caspase3 in a time- and dose-dependent manner in both cell lines. In addition, JMR-132 treatments decreased significantly the epidermal growth factor receptor (EGFR) level and the phosphorylation of Akt (p-Akt), suggesting that JMR-132 inhibits the EGFR-Akt pathway in ovarian cancer cells. More importantly, treatment of SKOV3 and CaOV3 cells with 100 nM JMR-132 attenuated proliferation and the antiapoptotic effect induced by EGF in both cell lines. After the knockdown of the expression of EGFR by siRNA, the antiproliferative effect of JMR-132 was abolished in SKOV3 and CaOV3 cells.
Conclusions: The present study demonstrates that the inhibitory effect of the GHRH antagonist JMR-132 on proliferation is due, in part, to an interference with the EGFR-Akt pathway in ovarian cancer cells.
Figures





Similar articles
-
Cellular mechanisms of growth inhibition of human endometrial cancer cell line by an antagonist of growth hormone-releasing hormone.Int J Oncol. 2008 Mar;32(3):593-601. Int J Oncol. 2008. PMID: 18292936
-
Impact of Axis of GHRH and GHRH Receptor on Cell Viability and Apoptosis of the Placental Choriocarcinoma Cell Line.Curr Mol Med. 2016;16(3):299-311. doi: 10.2174/1566524016666160225154040. Curr Mol Med. 2016. PMID: 26917260
-
Combination of GHRH antagonists and docetaxel shows experimental effectiveness for the treatment of triple-negative breast cancers.Oncol Rep. 2013 Jul;30(1):413-8. doi: 10.3892/or.2013.2435. Epub 2013 Apr 29. Oncol Rep. 2013. PMID: 23624870
-
GHRH and the prostate.Rev Endocr Metab Disord. 2025 Jun;26(3):467-481. doi: 10.1007/s11154-024-09922-9. Epub 2024 Nov 7. Rev Endocr Metab Disord. 2025. PMID: 39505776 Review.
-
Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer.Nat Clin Pract Endocrinol Metab. 2008 Jan;4(1):33-43. doi: 10.1038/ncpendmet0677. Nat Clin Pract Endocrinol Metab. 2008. PMID: 18084344 Review.
Cited by
-
Growth hormone-releasing hormone receptor antagonists inhibit human gastric cancer through downregulation of PAK1-STAT3/NF-κB signaling.Proc Natl Acad Sci U S A. 2016 Dec 20;113(51):14745-14750. doi: 10.1073/pnas.1618582114. Epub 2016 Dec 7. Proc Natl Acad Sci U S A. 2016. PMID: 27930339 Free PMC article.
-
Selective uptake of epidermal growth factor-conjugated gold nanoparticle (EGF-GNP) facilitates non-thermal plasma (NTP)-mediated cell death.Sci Rep. 2017 Sep 8;7(1):10971. doi: 10.1038/s41598-017-11292-z. Sci Rep. 2017. PMID: 28887524 Free PMC article.
-
Effects of GHRH and its analogues on the Vascular System.Rev Endocr Metab Disord. 2025 Jun;26(3):493-505. doi: 10.1007/s11154-024-09932-7. Epub 2024 Nov 21. Rev Endocr Metab Disord. 2025. PMID: 39570567 Review.
-
Growth Hormone-Releasing Hormone in Endothelial Inflammation.Endocrinology. 2022 Dec 19;164(2):bqac209. doi: 10.1210/endocr/bqac209. Endocrinology. 2022. PMID: 36503995 Free PMC article. Review.
-
Growth hormone-releasing hormone and its analogues in health and disease.Nat Rev Endocrinol. 2025 Mar;21(3):180-195. doi: 10.1038/s41574-024-01052-1. Epub 2024 Nov 13. Nat Rev Endocrinol. 2025. PMID: 39537825 Review.
References
-
- Gelato M. Growth hormone releasing hormone: clinical perspectives revisited. Endocrinologist. 2005;15:159–164. doi: 10.1097/01.ten.0000162232.25674.d6. - DOI
-
- Szepeshazi K, Schally AV, Groot K, Armatis P, Hebert F, Halmos G. Antagonists of growth hormone-releasing hormone (GH-RH) inhibit in vivo proliferation of experimental pancreatic cancers and decrease IGF-II levels in tumours. Eur J Cancer. 2000;36:128–136. doi: 10.1016/S0959-8049(99)00230-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

