Protective effect of budesonide/formoterol compared with formoterol, salbutamol and placebo on repeated provocations with inhaled AMP in patients with asthma: a randomised, double-blind, cross-over study
- PMID: 20509942
- PMCID: PMC2890647
- DOI: 10.1186/1465-9921-11-66
Protective effect of budesonide/formoterol compared with formoterol, salbutamol and placebo on repeated provocations with inhaled AMP in patients with asthma: a randomised, double-blind, cross-over study
Abstract
Background: The budesonide/formoterol combination is successfully used for fast relief of asthma symptoms in addition to its use as maintenance therapy. The temporarily increased corticosteroid dose during increasing inhaler use for symptom relief is likely to suppress any temporary increase in airway inflammation and may mitigate or prevent asthma exacerbations. The relative contribution of the budesonide and formoterol components to the improved asthma control is unclear.
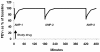
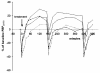
Methods: The acute protective effect of inhaled budesonide was tested in a model of temporarily increased airway inflammation with repeated indirect airway challenges, mimicking an acute asthma exacerbation. A randomised, double-blind, cross-over study design was used. Asthmatic patients (n = 17, mean FEV1 95% of predicted) who previously demonstrated a > or = 30% fall in forced expiratory volume in 1 second (FEV1) after inhaling adenosine 5'-monophosphate (AMP), were challenged on four consecutive test days, with the same dose of AMP (at 09:00, 12:00 and 16:00 hours). Within 1 minute of the maximal AMP-induced bronchoconstriction at 09:00 hours, the patients inhaled one dose of either budesonide/formoterol (160/4.5 microg), formoterol (4.5 microg), salbutamol (2 x 100 microg) or placebo. The protective effects of the randomised treatments were assessed by serial lung function measurements over the test day.
Results: In the AMP provocations at 3 and 7 hours after inhalation, the budesonide/formoterol combination provided a greater protective effect against AMP-induced bronchoconstriction compared with formoterol alone, salbutamol and placebo. In addition all three active treatments significantly increased FEV1 within 3 minutes of administration, at a time when inhaled AMP had induced the 30% fall in FEV1.
Conclusions: A single dose of budesonide/formoterol provided a greater protective effect against inhaled AMP-induced bronchoconstriction than formoterol alone, both at 3 and at 7 hours after inhalation. The acute protection against subsequent bronchoconstrictor stimuli such as inhaled AMP and the rapid reversal of airway obstruction supports the use of budesonide/formoterol for both relief and prevention in the treatment of asthma.
Trial registration: ClinicalTrials.gov NCT00272753.
Figures


Similar articles
-
Onset of relief of dyspnoea with budesonide/formoterol or salbutamol following methacholine-induced severe bronchoconstriction in adults with asthma: a double-blind, placebo-controlled study.Respir Res. 2006 Dec 4;7(1):141. doi: 10.1186/1465-9921-7-141. Respir Res. 2006. PMID: 17144916 Free PMC article. Clinical Trial.
-
Budesonide/formoterol in a single inhaler rapidly relieves methacholine-induced moderate-to-severe bronchoconstriction.Pulm Pharmacol Ther. 2004;17(2):89-95. doi: 10.1016/j.pupt.2003.11.001. Pulm Pharmacol Ther. 2004. PMID: 15123230 Clinical Trial.
-
Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta2 agonists.Thorax. 2001 Jul;56(7):529-35. doi: 10.1136/thorax.56.7.529. Thorax. 2001. PMID: 11413351 Free PMC article. Clinical Trial.
-
Treatment of persistent asthma with Symbicort (budesonide/formoterol inhalation aerosol): an inhaled corticosteroid and long-acting beta2-adrenergic agonist in one pressurized metered-dose inhaler.J Asthma. 2010 May;47(4):447-59. doi: 10.3109/02770901003725684. J Asthma. 2010. PMID: 20528601 Review.
-
Single maintenance and reliever therapy (SMART) of asthma: a critical appraisal.Thorax. 2010 Aug;65(8):747-52. doi: 10.1136/thx.2009.128504. Epub 2010 Jun 27. Thorax. 2010. PMID: 20581409 Free PMC article. Review.
Cited by
-
Rapid effects of extrafine beclomethasone dipropionate/formoterol fixed combination inhaler on airway inflammation and bronchoconstriction in asthma: a randomised controlled trial.BMC Pulm Med. 2011 Dec 21;11:60. doi: 10.1186/1471-2466-11-60. BMC Pulm Med. 2011. PMID: 22188731 Free PMC article. Clinical Trial.
-
Controversies in Allergy: Should I Combine an ICS With a SABA or With Formoterol for Reliever Therapy?J Allergy Clin Immunol Pract. 2025 Jul;13(7):1599-1604. doi: 10.1016/j.jaip.2025.04.016. Epub 2025 Apr 16. J Allergy Clin Immunol Pract. 2025. PMID: 40250558 Review.
References
-
- Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA); 2008. Global Initiative for Asthma.http://www.ginasthma.org

