High cell density cultivation and recombinant protein production with Escherichia coli in a rocking-motion-type bioreactor
- PMID: 20509968
- PMCID: PMC2891675
- DOI: 10.1186/1475-2859-9-42
High cell density cultivation and recombinant protein production with Escherichia coli in a rocking-motion-type bioreactor
Abstract
Background: Single-use rocking-motion-type bag bioreactors provide advantages compared to standard stirred tank bioreactors by decreased contamination risks, reduction of cleaning and sterilization time, lower investment costs, and simple and cheaper validation. Currently, they are widely used for cell cultures although their use for small and medium scale production of recombinant proteins with microbial hosts might be very attractive. However, the utilization of rocking- or wave-induced motion-type bioreactors for fast growing aerobic microbes is limited because of their lower oxygen mass transfer rate. A conventional approach to reduce the oxygen demand of a culture is the fed-batch technology. New developments, such as the BIOSTAT CultiBag RM system pave the way for applying advanced fed-batch control strategies also in rocking-motion-type bioreactors. Alternatively, internal substrate delivery systems such as EnBase Flo provide an opportunity for adopting simple to use fed-batch-type strategies to shaken cultures. Here, we investigate the possibilities which both strategies offer in view of high cell density cultivation of E. coli and recombinant protein production.
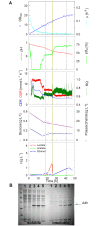
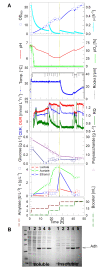
Results: Cultivation of E. coli in the BIOSTAT CultiBag RM system in a conventional batch mode without control yielded an optical density (OD(600)) of 3 to 4 which is comparable to shake flasks. The culture runs into oxygen limitation. In a glucose limited fed-batch culture with an exponential feed and oxygen pulsing, the culture grew fully aerobically to an OD(600) of 60 (20 g L(-1) cell dry weight). By the use of an internal controlled glucose delivery system, EnBase Flo, OD(600) of 30 (10 g L(-1) cell dry weight) is obtained without the demand of computer controlled external nutrient supply. EnBase Flo also worked well in the CultiBag RM system with a recombinant E. coli RB791 strain expressing a heterologous alcohol dehydrogenase (ADH) to very high levels, indicating that the enzyme based feed supply strategy functions well for recombinant protein production also in a rocking-motion-type bioreactor.
Conclusions: Rocking-motion-type bioreactors may provide an interesting alternative to standard cultivation in bioreactors for cultivation of bacteria and recombinant protein production. The BIOSTAT Cultibag RM system with the single-use sensors and advanced control system paves the way for the fed-batch technology also to rocking-motion-type bioreactors. It is possible to reach cell densities which are far above shake flasks and typical for stirred tank reactors with the improved oxygen transfer rate. For more simple applications the EnBase Flo method offers an easy and robust solution for rocking-motion-systems which do not have such advanced control possibilities.
Figures

Similar articles
-
Glucose-limited high cell density cultivations from small to pilot plant scale using an enzyme-controlled glucose delivery system.N Biotechnol. 2012 Jan 15;29(2):235-42. doi: 10.1016/j.nbt.2011.11.004. Epub 2011 Nov 9. N Biotechnol. 2012. PMID: 22100433
-
A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures.Microb Cell Fact. 2010 Feb 19;9:11. doi: 10.1186/1475-2859-9-11. Microb Cell Fact. 2010. PMID: 20167131 Free PMC article.
-
The fed-batch principle for the molecular biology lab: controlled nutrient diets in ready-made media improve production of recombinant proteins in Escherichia coli.Microb Cell Fact. 2016 Jun 17;15(1):110. doi: 10.1186/s12934-016-0513-8. Microb Cell Fact. 2016. PMID: 27317421 Free PMC article. Review.
-
Growth and recombinant protein expression with Escherichia coli in different batch cultivation media.Appl Microbiol Biotechnol. 2011 Apr;90(1):69-76. doi: 10.1007/s00253-010-3036-y. Epub 2010 Dec 23. Appl Microbiol Biotechnol. 2011. PMID: 21181153
-
Bag bioreactor based on wave-induced motion: characteristics and applications.Adv Biochem Eng Biotechnol. 2009;115:55-87. doi: 10.1007/10_2008_15. Adv Biochem Eng Biotechnol. 2009. PMID: 19373453 Review.
Cited by
-
Changes in growth, lanthanide binding, and gene expression in Pseudomonas alloputida KT2440 in response to light and heavy lanthanides.mSphere. 2024 Oct 29;9(10):e0068524. doi: 10.1128/msphere.00685-24. Epub 2024 Sep 18. mSphere. 2024. PMID: 39291981 Free PMC article.
-
Conversion of Escherichia coli to Generate All Biomass Carbon from CO2.Cell. 2019 Nov 27;179(6):1255-1263.e12. doi: 10.1016/j.cell.2019.11.009. Cell. 2019. PMID: 31778652 Free PMC article.
-
Computational Modelling of Metabolic Burden and Substrate Toxicity in Escherichia coli Carrying a Synthetic Metabolic Pathway.Microorganisms. 2019 Nov 11;7(11):553. doi: 10.3390/microorganisms7110553. Microorganisms. 2019. PMID: 31718036 Free PMC article.
-
Effect of culture medium, host strain and oxygen transfer on recombinant Fab antibody fragment yield and leakage to medium in shaken E. coli cultures.Microb Cell Fact. 2013 Jul 29;12:73. doi: 10.1186/1475-2859-12-73. Microb Cell Fact. 2013. PMID: 23895637 Free PMC article.
-
The important versus the exciting: reining contradictions in contemporary biotechnology.Microb Biotechnol. 2019 Jan;12(1):32-34. doi: 10.1111/1751-7915.13348. Epub 2018 Dec 3. Microb Biotechnol. 2019. PMID: 30508281 Free PMC article. No abstract available.
References
-
- Eibl R, Eibl D. Application of Disposable Bag-Bioreactors in Tissue Engineering and for the Production of Therapeutic Agents. Adv Biochem Eng Biotechnol. 2009;112:183–207. - PubMed
-
- Eibl R, Werner S, Eibl D. Disposable bioreactors for plant liquid cultures at Litre-scale. Engineering in Life Sciences. 2009;9:156–164. doi: 10.1002/elsc.200800102. - DOI
-
- Eibl R, Werner S, Eibl D. Bag Bioreactor Based on Wave-Induced Motion: Characteristics and Applications. Adv Biochem Eng Biotechnol. 2010;115:55–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

