Quantitative analysis of the effect of tubulin isotype expression on sensitivity of cancer cell lines to a set of novel colchicine derivatives
- PMID: 20509970
- PMCID: PMC2890610
- DOI: 10.1186/1476-4598-9-131
Quantitative analysis of the effect of tubulin isotype expression on sensitivity of cancer cell lines to a set of novel colchicine derivatives
Abstract
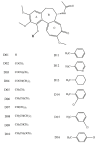
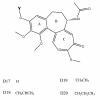
Background: A maximum entropy approach is proposed to predict the cytotoxic effects of a panel of colchicine derivatives in several human cancer cell lines. Data was obtained from cytotoxicity assays performed with 21 drug molecules from the same family of colchicine compounds and correlate these results with independent tubulin isoform expression measurements for several cancer cell lines. The maximum entropy method is then used in conjunction with computed relative binding energy values for each of the drug molecules against tubulin isotypes to which these compounds bind with different affinities.
Results: We have found by using our analysis that alphabetaI and alphabetaIII tubulin isoforms are the most important isoforms in establishing predictive response of cancer cell sensitivity to colchicine derivatives. However, since alphabetaI tubulin is widely distributed in the human body, targeting it would lead to severe adverse side effects. Consequently, we have identified tubulin isotype alphabetaIII as the most important molecular target for inhibition of microtubule polymerization and hence cancer cell cytotoxicity. Tubulin isotypes alphabetaI and alphabetaII are concluded to be secondary targets.
Conclusions: The benefit of being able to correlate expression levels of specific tubulin isotypes and the resultant cell death effect is that it will enable us to better understand the origin of drug resistance and hence design optimal structures for the elimination of cancer cells. The conclusion of the study described herein identifies tubulin isotype alphabetaIII as a target for optimized chemotherapy drug design.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

