Reptin is required for the transcription of telomerase reverse transcriptase and over-expressed in gastric cancer
- PMID: 20509972
- PMCID: PMC2887797
- DOI: 10.1186/1476-4598-9-132
Reptin is required for the transcription of telomerase reverse transcriptase and over-expressed in gastric cancer
Abstract
Background: Telomerase is activated in oncogenesis, which confers an immortal phenotype to cancer cells. The AAA + ATPase Reptin is required for telomerase biogenesis by maintaining telomerase RNA (hTER) stability and is aberrantly expressed in certain cancers. Given its role in chromatin remodeling and transcription regulation, we determined the effect of Reptin on the transcription of the telomerase reverse transcriptase (hTERT) gene, a key component of the telomerase complex and its expression in gastric cancer.
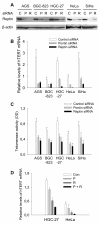
Results: Knocking down Reptin or its partner Pontin using small interfering RNA in gastric and cervical cancer cells led to significant decreases in hTERT mRNA, but hTERT promoter activity was inhibited in only Reptin-depleted cells. Reptin interacted with the c-MYC oncoprotein and its stimulatory effect on the hTERTpromoter was significantly dependent on functional E-boxes in the promoter. Moreover, Reptin bound to the hTERT proximal promoter and the loss of the Reptin occupancy led to dissociation of c-MYC from the hTERT promoter in Reptin-depleted cells. Reptin inhibition dramatically impaired clonogenic potential of gastric cancer cells by inducing cell growtharrest and over-expression of Reptin was observed in primary gastric cancer specimens.
Conclusions: The hTERT gene is a direct target of Reptin, and hTERT transcription requires constitutive expression of Reptin and its cooperation with c-MYC. Thus, Reptin regulates telomerase at two different levels. This finding, together with the requirementof Reptin for the clonogenic potential of cancer cells and its over-expression in gastriccancer and other solid tumors, suggests that Reptin may be a putative therapeutic target.
Figures



References
-
- Liu JP. Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J. 1999;13:2091–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

