Retrospective analyses of the bottleneck in purification of eukaryotic proteins from Escherichia coli as affected by molecular weight, cysteine content and isoelectric point
- PMID: 20510014
- PMCID: PMC4546832
- DOI: 10.5483/bmbrep.2010.43.5.319
Retrospective analyses of the bottleneck in purification of eukaryotic proteins from Escherichia coli as affected by molecular weight, cysteine content and isoelectric point
Abstract
Experimental bioinformatics data obtained from an E. coli cell-based eukaryotic protein purification experiment were analyzed in order to identify any bottleneck as well as the factors affecting the target purification. All targets were expressed as His-tagged maltose-binding protein (MBP) fusion constructs and were initially purified by immobilized metal affinity chromatography (IMAC). The targets were subsequently separated from the His-tagged MBP through TEV protease cleavage followed by a second IMAC isolation. Of the 743 total purification trials, 342 yielded more than 3 mg of target proteins for structural studies. The major reason for failure of target purification was poor TEV proteolysis. The overall success rate for target purification decreased linearly as cysteine content or isoelectric point (pI) of the target increased. This pattern of pI versus overall success rate strongly suggests that pI should be incorporated into target scoring criteria with a threshold value.
Figures
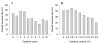
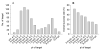
Similar articles
-
Expression and purification of soluble His(6)-tagged TEV protease.Methods Mol Biol. 2009;498:297-307. doi: 10.1007/978-1-59745-196-3_19. Methods Mol Biol. 2009. PMID: 18988033
-
High yield purification of nanobodies from the periplasm of E. coli as fusions with the maltose binding protein.Protein Expr Purif. 2013 Sep;91(1):42-8. doi: 10.1016/j.pep.2013.07.001. Epub 2013 Jul 13. Protein Expr Purif. 2013. PMID: 23856605
-
Removal of Affinity Tags with TEV Protease.Methods Mol Biol. 2017;1586:221-230. doi: 10.1007/978-1-4939-6887-9_14. Methods Mol Biol. 2017. PMID: 28470608 Free PMC article.
-
Secretion and proteolysis of heterologous proteins fused to the Escherichia coli maltose binding protein in Pichia pastoris.Protein Expr Purif. 2010 Jul;72(1):113-24. doi: 10.1016/j.pep.2010.03.004. Epub 2010 Mar 15. Protein Expr Purif. 2010. PMID: 20230898 Free PMC article.
-
Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner.Methods Mol Biol. 2011;705:259-74. doi: 10.1007/978-1-61737-967-3_16. Methods Mol Biol. 2011. PMID: 21125392 Review.
Cited by
-
Two legume fatty acid amide hydrolase isoforms with distinct preferences for microbial- and plant-derived acylamides.Sci Rep. 2023 May 9;13(1):7486. doi: 10.1038/s41598-023-34754-z. Sci Rep. 2023. PMID: 37161076 Free PMC article.
References
-
- Lesley SA. High-Throughput proteomics: protein expression and purification in the postgenomic world. Protein Expr. Purif. 2001;122:159–164. - PubMed
-
- Gathmann S, Rupprecht E, Schneider D. High level expression of a protein precursor for functional studies. BMB Reports. 2006;39:717–721. - PubMed
-
- Chrunyk BA, Evans J, Lillquist J, Young P, Wetzel R. Inclusion body formation and protein stability in sequence variants of interleukin-1 beta. J. Biol. Chem. 1993;268:18053–18061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

