Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis
- PMID: 20510279
- PMCID: PMC3046810
- DOI: 10.1016/S1473-3099(10)70071-2
Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis
Abstract
WHO has previously recommended sputum-smear examination at the end of the second month of treatment in patients with recently diagnosed pulmonary tuberculosis, and, if positive, extension of the intensive therapy phase. We did a systematic review and meta-analysis to assess the accuracy of a positive sputum smear or culture during treatment for predicting failure or relapse in pulmonary tuberculosis. We searched PubMed, Embase, and the Cochrane Library for studies published in English through December, 2009. We included randomised controlled trials, cohort, and case-control studies of previously untreated pulmonary tuberculosis patients who had received a standardised regimen with rifampicin in the initial phase. Accuracy results were summarised in forest plots and pooled by use of a hierarchical regression approach. 15 papers (28 studies) met the inclusion criteria. The pooled sensitivities for both 2-month smear (24% [95% CI 12-42%], six studies) and culture (40% [95% CI 25-56%], four studies) to predict relapse were low. Corresponding specificities (85% [95% CI 72-90%] and 85% [95% CI 77-91%]) were higher, but modest. For failure, 2-month smear (seven studies) had low sensitivity (57% [95% CI 41-73%]) and higher, although modest, specificity (81% [95% CI 72-87%]). Both sputum-smear microscopy and mycobacterial culture during tuberculosis treatment have low sensitivity and modest specificity for predicting failure and relapse. Although we pooled a diverse group of patients, the individual studies had similar performance characteristics. Better predictive markers are needed.
2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest
We declare that we have no conflicts of interest.
Figures
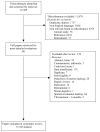
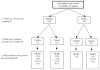


Comment in
-
Sputum monitoring during tuberculosis treatment for predicting outcome.Lancet Infect Dis. 2011 Mar;11(3):160; author reply 160-1. doi: 10.1016/S1473-3099(11)70043-3. Lancet Infect Dis. 2011. PMID: 21371650 No abstract available.
References
-
- World Health Organization. Global tuberculosis control: epidemiology, planning, financing: WHO report 2009. Geneva: World Health Organization; 2009.
-
- World Health Organization. Treatment of Tuberculosis: Guidelines for National Programmes. WHO Global Tuberculosis Programme; 2003.
-
- Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–75. - PubMed
-
- Sterling TR, Alwood K, Gachuhi R, et al. Relapse rates after short-course (6-month) treatment of tuberculosis in HIV-infected and uninfected persons. AIDS. 1999;13:1899–904. - PubMed
-
- Five-year follow-up of a controlled trial of five 6-month regimens of chemotherapy for pulmonary tuberculosis. Hong Kong Chest Service/British Medical Research Council. Am Rev Respir Dis. 1987;136:1339–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

