Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex
- PMID: 20510857
- PMCID: PMC2894581
- DOI: 10.1016/j.neuron.2010.04.038
Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex
Abstract
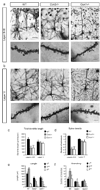
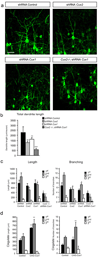
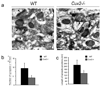
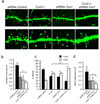
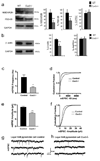
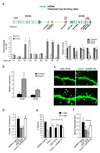
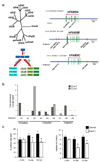
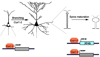
Dendrite branching and spine formation determines the function of morphologically distinct and specialized neuronal subclasses. However, little is known about the programs instructing specific branching patterns in vertebrate neurons and whether such programs influence dendritic spines and synapses. Using knockout and knockdown studies combined with morphological, molecular, and electrophysiological analysis, we show that the homeobox Cux1 and Cux2 are intrinsic and complementary regulators of dendrite branching, spine development, and synapse formation in layer II-III neurons of the cerebral cortex. Cux genes control the number and maturation of dendritic spines partly through direct regulation of the expression of Xlr3b and Xlr4b, chromatin remodeling genes previously implicated in cognitive defects. Accordingly, abnormal dendrites and synapses in Cux2(-/-) mice correlate with reduced synaptic function and defects in working memory. These demonstrate critical roles of Cux in dendritogenesis and highlight subclass-specific mechanisms of synapse regulation that contribute to the establishment of cognitive circuits.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Arion D, Unger T, Lewis DA, Mirnics K. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. The European journal of neuroscience. 2007;25:1843–1854. - PubMed
-
- Ballesteros-Yanez I, Benavides-Piccione R, Elston GN, Yuste R, DeFelipe J. Density and morphology of dendritic spines in mouse neocortex. Neuroscience. 2006;138:403–409. - PubMed
-
- Benavides-Piccione R, Hamzei-Sichani F, Ballesteros-Yanez I, DeFelipe J, Yuste R. Dendritic size of pyramidal neurons differs among mouse cortical regions. Cereb Cortex. 2006;16:990–1001. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Current opinion in neurobiology. 2007;17:381–386. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

