Hematopoietic origin of pathological grooming in Hoxb8 mutant mice
- PMID: 20510925
- PMCID: PMC2894573
- DOI: 10.1016/j.cell.2010.03.055
Hematopoietic origin of pathological grooming in Hoxb8 mutant mice
Abstract
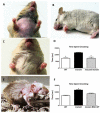
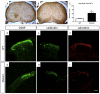
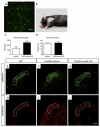
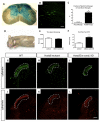
Mouse Hoxb8 mutants show unexpected behavior manifested by compulsive grooming and hair removal, similar to behavior in humans with the obsessive-compulsive disorder spectrum disorder trichotillomania. As Hox gene disruption often has pleiotropic effects, the root cause of this behavioral deficit was unclear. Here we report that, in the brain, Hoxb8 cell lineage exclusively labels bone marrow-derived microglia. Furthermore, transplantation of wild-type bone marrow into Hoxb8 mutant mice rescues their pathological phenotype. It has been suggested that the grooming dysfunction results from a nociceptive defect, also exhibited by Hoxb8 mutant mice. However, bone marrow transplant experiments and cell type-specific disruption of Hoxb8 reveal that these two phenotypes are separable, with the grooming phenotype derived from the hematopoietic lineage and the sensory defect derived from the spinal cord cells. Immunological dysfunctions have been associated with neuropsychiatric disorders, but the causative relationships are unclear. In this mouse, a distinct compulsive behavioral disorder is associated with mutant microglia.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



Comment in
-
A bone to pick with compulsive behavior.Cell. 2010 May 28;141(5):752-4. doi: 10.1016/j.cell.2010.05.010. Cell. 2010. PMID: 20510922
References
-
- Aldridge JW, Berridge KC, Herman M, Zimmer L. Neuronal coding of serial order: syntax of grooming in the neostriatum. Psychol Sci. 1993;4:391–395.
-
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. - PubMed
-
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. - PubMed
-
- Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behav Brain Res. 1989;33:241–253. - PubMed
-
- Berridge KC, Fentress JC, Parr H. Natural syntax rules control action sequence of rats. Behav Brain Res. 1987;23:59–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

