A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain
- PMID: 20510930
- PMCID: PMC2905675
- DOI: 10.1016/j.cell.2010.03.052
A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain
Abstract
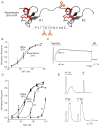
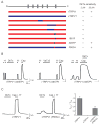
Toxins have evolved to target regions of membrane ion channels that underlie ligand binding, gating, or ion permeation, and have thus served as invaluable tools for probing channel structure and function. Here, we describe a peptide toxin from the Earth Tiger tarantula that selectively and irreversibly activates the capsaicin- and heat-sensitive channel, TRPV1. This high-avidity interaction derives from a unique tandem repeat structure of the toxin that endows it with an antibody-like bivalency. The "double-knot" toxin traps TRPV1 in the open state by interacting with residues in the presumptive pore-forming region of the channel, highlighting the importance of conformational changes in the outer pore region of TRP channels during activation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Bulaj G, Olivera BM. Folding of conotoxins: formation of the native disulfide bridges during chemical synthesis and biosynthesis of Conus peptides. Antioxid Redox Signal. 2008;10:141–155. - PubMed
-
- Catterall WA, Cestele S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. - PubMed
-
- Cestele S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA. Voltage sensor-trapping: enhanced activation of sodium channels by beta-scorpion toxin bound to the S3-S4 loop in domain II. Neuron. 1998;21:919–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

