Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization
- PMID: 20510932
- PMCID: PMC2909748
- DOI: 10.1016/j.cell.2010.03.053
Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization
Abstract
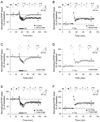
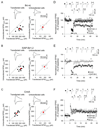
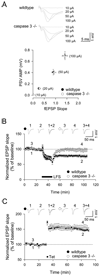
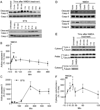
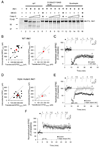
NMDA receptor-dependent synaptic modifications, such as long-term potentiation (LTP) and long-term depression (LTD), are essential for brain development and function. LTD occurs mainly by the removal of AMPA receptors from the postsynaptic membrane, but the underlying molecular mechanisms remain unclear. Here, we show that activation of caspase-3 via mitochondria is required for LTD and AMPA receptor internalization in hippocampal neurons. LTD and AMPA receptor internalization are blocked by peptide inhibitors of caspase-3 and -9. In hippocampal slices from caspase-3 knockout mice, LTD is abolished whereas LTP remains normal. LTD is also prevented by overexpression of the anti-apoptotic proteins XIAP or Bcl-xL, and by a mutant Akt1 protein that is resistant to caspase-3 proteolysis. NMDA receptor stimulation that induces LTD transiently activates caspase-3 in dendrites, without causing cell death. These data indicate an unexpected causal link between the molecular mechanisms of apoptosis and LTD.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Comment in
-
For synapses, it's depression not death.Cell. 2010 May 28;141(5):750-2. doi: 10.1016/j.cell.2010.05.013. Cell. 2010. PMID: 20510921
-
Synaptic plasticity: depress or die.Nat Rev Neurosci. 2010 Jul;11(7):455. doi: 10.1038/nrn2875. Nat Rev Neurosci. 2010. PMID: 21467989 No abstract available.
References
-
- Anderson WWaC, GL The LTP Program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. - PubMed
-
- Barry MF, Vickery RM, Bolsover SR, Bindman LJ. Intracellular studies of heterosynaptic long-term depression (LTD) in CA1 of hippocampal slices. Hippocampus. 1996;6:3–8. - PubMed
-
- Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

