Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study
- PMID: 20511019
- PMCID: PMC7138079
- DOI: 10.1016/S0140-6736(10)60357-1
Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study
Abstract
Background: We previously showed that small interfering RNAs (siRNAs) targeting the Zaire Ebola virus (ZEBOV) RNA polymerase L protein formulated in stable nucleic acid-lipid particles (SNALPs) completely protected guineapigs when administered shortly after a lethal ZEBOV challenge. Although rodent models of ZEBOV infection are useful for screening prospective countermeasures, they are frequently not useful for prediction of efficacy in the more stringent non-human primate models. We therefore assessed the efficacy of modified non-immunostimulatory siRNAs in a uniformly lethal non-human primate model of ZEBOV haemorrhagic fever.
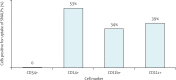
Methods: A combination of modified siRNAs targeting the ZEBOV L polymerase (EK-1 mod), viral protein (VP) 24 (VP24-1160 mod), and VP35 (VP35-855 mod) were formulated in SNALPs. A group of macaques (n=3) was given these pooled anti-ZEBOV siRNAs (2 mg/kg per dose, bolus intravenous infusion) after 30 min, and on days 1, 3, and 5 after challenge with ZEBOV. A second group of macaques (n=4) was given the pooled anti-ZEBOV siRNAs after 30 min, and on days 1, 2, 3, 4, 5, and 6 after challenge with ZEBOV.
Findings: Two (66%) of three rhesus monkeys given four postexposure treatments of the pooled anti-ZEBOV siRNAs were protected from lethal ZEBOV infection, whereas all macaques given seven postexposure treatments were protected. The treatment regimen in the second study was well tolerated with minor changes in liver enzymes that might have been related to viral infection.
Interpretation: This complete postexposure protection against ZEBOV in non-human primates provides a model for the treatment of ZEBOV-induced haemorrhagic fever. These data show the potential of RNA interference as an effective postexposure treatment strategy for people infected with Ebola virus, and suggest that this strategy might also be useful for treatment of other emerging viral infections.
Funding: Defense Threat Reduction Agency.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Are we any closer to combating Ebola infections?Lancet. 2010 May 29;375(9729):1850-2. doi: 10.1016/S0140-6736(10)60597-1. Lancet. 2010. PMID: 20511001 Free PMC article. No abstract available.
References
-
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams and Wilkins; Philadelphia: 2006. pp. 1409–1448.
-
- Sanchez A, Kiley MP, Holloway BP, Auperin DD. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993;29:215–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

