Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex
- PMID: 20511283
- PMCID: PMC2877906
- DOI: 10.1093/brain/awq112
Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex
Abstract
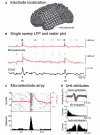
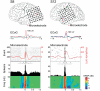
Epileptic cortex is characterized by paroxysmal electrical discharges. Analysis of these interictal discharges typically manifests as spike-wave complexes on electroencephalography, and plays a critical role in diagnosing and treating epilepsy. Despite their fundamental importance, little is known about the neurophysiological mechanisms generating these events in human focal epilepsy. Using three different systems of microelectrodes, we recorded local field potentials and single-unit action potentials during interictal discharges in patients with medically intractable focal epilepsy undergoing diagnostic workup for localization of seizure foci. We studied 336 single units in 20 patients. Ten different cortical areas and the hippocampus, including regions both inside and outside the seizure focus, were sampled. In three of these patients, high density microelectrode arrays simultaneously recorded between 43 and 166 single units from a small (4 mm x 4 mm) patch of cortex. We examined how the firing rates of individual neurons changed during interictal discharges by determining whether the firing rate during the event was the same, above or below a median baseline firing rate estimated from interictal discharge-free periods (Kruskal-Wallis one-way analysis, P<0.05). Only 48% of the recorded units showed such a modulation in firing rate within 500 ms of the discharge. Units modulated during the discharge exhibited significantly higher baseline firing and bursting rates than unmodulated units. As expected, many units (27% of the modulated population) showed an increase in firing rate during the fast segment of the discharge (+ or - 35 ms from the peak of the discharge), while 50% showed a decrease during the slow wave. Notably, in direct contrast to predictions based on models of a pure paroxysmal depolarizing shift, 7.7% of modulated units recorded in or near the seizure focus showed a decrease in activity well ahead (0-300 ms) of the discharge onset, while 12.2% of units increased in activity in this period. No such pre-discharge changes were seen in regions well outside the seizure focus. In many recordings there was also a decrease in broadband field potential activity during this same pre-discharge period. The different patterns of interictal discharge-modulated firing were classified into more than 15 different categories. This heterogeneity in single unit activity was present within small cortical regions as well as inside and outside the seizure onset zone, suggesting that interictal epileptiform activity in patients with epilepsy is not a simple paroxysm of hypersynchronous excitatory activity, but rather represents an interplay of multiple distinct neuronal types within complex neuronal networks.
Figures
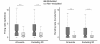



References
-
- Altafullah I, Halgren E, Stapleton JM, Crandall PH. Interictal spike-wave complexes in the human medial temporal lobe: typical topography and comparisons with cognitive potentials. Electroencephalogr Clin Neurophysiol. 1986;63:503–16. - PubMed
-
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. - PubMed
-
- Babb TL, Carr E, Crandall PH. Analysis of extracellular firing patterns of deep temporal lobe structures in man. Electroencephalogr Clin Neurophysiol. 1973;34:247–57. - PubMed
-
- Babb TL, Crandall PH. Epileptogenesis of human limbic neurons in psychomotor epileptics. Electroencephalogr Clin Neurophysiol. 1976;40:225–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

