(Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor
- PMID: 20511320
- PMCID: PMC3227994
- DOI: 10.1634/theoncologist.2009-0274
(Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor
Abstract
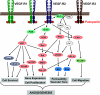
Pazopanib is a recently approved, novel tyrosine kinase inhibitor specifically designed to impair angiogenesis by abrogating vascular endothelial growth factor receptor 2 (VEGFR-2) to exert its function. Pazopanib inhibits VEGF-induced endothelial cell proliferation in vitro and angiogenesis in vivo and demonstrates antitumor activity in mouse models. Furthermore, the pazopanib concentration resulting in maximal inhibition of VEGFR-2 phosphorylation in vivo was in line with the steady-state concentration required to inhibit growth of tumor xenografts, suggesting that pazopanib's mechanism of action is indeed through VEGFR-2 inhibition. In a phase I trial, a generally well-tolerated dose was identified at which the majority of patients achieved pazopanib plasma concentrations above the concentration required for maximal in vivo inhibition of VEGFR-2 phosphorylation in preclinical models. Administered as monotherapy, evidence of antitumor activity was observed in phase II studies in several tumor types, including soft tissue sarcoma, renal cell cancer (RCC), ovarian cancer, and non-small cell lung cancer. Recently, the U.S. Food and Drug Administration granted approval for treatment with pazopanib in patients with RCC based on the longer progression-free survival time observed with this agent in a placebo-controlled, randomized trial. This review summarizes the preclinical and clinical pharmacokinetics and pharmacodynamics of pazopanib, as well as data on clinical activity, that ultimately resulted in its recent approval.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures

Similar articles
-
Pazopanib for the treatment of metastatic renal cell carcinoma.Clin Ther. 2012 Mar;34(3):511-20. doi: 10.1016/j.clinthera.2012.01.014. Epub 2012 Feb 16. Clin Ther. 2012. PMID: 22341567 Review.
-
Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity.Mol Cancer Ther. 2007 Jul;6(7):2012-21. doi: 10.1158/1535-7163.MCT-07-0193. Mol Cancer Ther. 2007. PMID: 17620431
-
Combination therapy with pazopanib and tivantinib modulates VEGF and c-MET levels in refractory advanced solid tumors.Invest New Drugs. 2021 Dec;39(6):1577-1586. doi: 10.1007/s10637-021-01138-x. Epub 2021 Jun 28. Invest New Drugs. 2021. PMID: 34180036 Free PMC article. Clinical Trial.
-
Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer.Mol Cancer Ther. 2010 Apr;9(4):985-95. doi: 10.1158/1535-7163.MCT-09-0967. Epub 2010 Apr 6. Mol Cancer Ther. 2010. PMID: 20371710 Free PMC article.
-
Pazopanib in ovarian cancer.Expert Rev Anticancer Ther. 2015;15(9):995-1005. doi: 10.1586/14737140.2015.1081383. Epub 2015 Aug 20. Expert Rev Anticancer Ther. 2015. PMID: 26296187 Review.
Cited by
-
Phase II Study of Pazopanib and Paclitaxel in Patients With Refractory Urothelial Cancer.Clin Genitourin Cancer. 2016 Oct;14(5):432-437. doi: 10.1016/j.clgc.2016.03.011. Epub 2016 Mar 12. Clin Genitourin Cancer. 2016. PMID: 27068017 Free PMC article. Clinical Trial.
-
Clinical characteristics and treatment outcomes in six cases of malignant tenosynovial giant cell tumor: initial experience of molecularly targeted therapy.BMC Cancer. 2018 Dec 29;18(1):1296. doi: 10.1186/s12885-018-5188-6. BMC Cancer. 2018. PMID: 30594158 Free PMC article.
-
Pazopanib, a Receptor Tyrosine Kinase Inhibitor, Suppresses Tumor Growth through Angiogenesis in Dedifferentiated Liposarcoma Xenograft Models.Transl Oncol. 2014 Dec;7(6):665-71. doi: 10.1016/j.tranon.2014.09.007. Transl Oncol. 2014. PMID: 25500074 Free PMC article.
-
Pursuing Precision: Receptor Tyrosine Kinase Inhibitors for Treatment of Pediatric Solid Tumors.Cancers (Basel). 2021 Jul 14;13(14):3531. doi: 10.3390/cancers13143531. Cancers (Basel). 2021. PMID: 34298746 Free PMC article. Review.
-
The role of RB1 alteration and 4q12 amplification in IDH-WT glioblastoma.Neurooncol Adv. 2021 Mar 31;3(1):vdab050. doi: 10.1093/noajnl/vdab050. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 34131647 Free PMC article.
References
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. - PubMed
-
- Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–5414. - PubMed
-
- Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: Its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–673. - PubMed
-
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

