Proteomics profiling of Madin-Darby canine kidney plasma membranes reveals Wnt-5a involvement during oncogenic H-Ras/TGF-beta-mediated epithelial-mesenchymal transition
- PMID: 20511395
- PMCID: PMC3033669
- DOI: 10.1074/mcp.M110.001131
Proteomics profiling of Madin-Darby canine kidney plasma membranes reveals Wnt-5a involvement during oncogenic H-Ras/TGF-beta-mediated epithelial-mesenchymal transition
Abstract
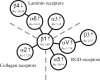
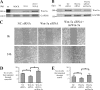
Epithelial-mesenchymal transition (EMT) describes a process whereby polarized epithelial cells with restricted migration transform into elongated spindle-shaped mesenchymal cells with enhanced motility and invasiveness. Although there are some molecular markers for this process, including the down-regulation of E-cadherin, our understanding of plasma membrane (PM) and associated proteins involved in EMT is limited. To specifically explore molecular alterations occurring at the PM, we used the cationic colloidal silica isolation technique to purify PM fractions from epithelial Madin-Darby canine kidney cells during Ras/TGF-β-mediated EMT. Proteins in the isolated membrane fractions were separated by one-dimensional SDS-PAGE and subjected to nano-LC-MS/MS-based protein identification. In this study, the first membrane protein analysis of an EMT model, we identified 805 proteins and determined their differential expression using label-free spectral counting. These data reveal that Madin-Darby canine kidney cells switch from cadherin-mediated to integrin-mediated adhesion following Ras/TGF-β-mediated EMT. Thus, during the EMT process, E-cadherin, claudin 4, desmoplakin, desmoglein-2, and junctional adhesion molecule A were down-regulated, whereas integrins α6β1, α3β1, α2β1, α5β1, αVβ1, and αVβ3 along with their extracellular ligands collagens I and V and fibronectin had increased expression levels. Conspicuously, Wnt-5a expression was elevated in cells undergoing EMT, and transient Wnt-5a siRNA silencing attenuated both cell migration and invasion in these cells. Furthermore, Wnt-5a expression suppressed canonical Wnt signaling induced by Wnt-3a. Wnt-5a may act through the planar cell polarity pathway of the non-canonical Wnt signaling pathway as several of the components and modulators (Wnt-5a, -5b, frizzled 6, collagen triple helix repeat-containing protein 1, tyrosine-protein kinase 7, RhoA, Rac, and JNK) were found to be up-regulated during Ras/TGF-β-mediated EMT.
Figures



References
-
- Kang Y., Massagué J. (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118, 277–279 - PubMed
-
- Thiery J. P. (2002) Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 - PubMed
-
- Thiery J. P. (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 - PubMed
-
- Hugo H., Ackland M. L., Blick T., Lawrence M. G., Clements J. A., Williams E. D., Thompson E. W. (2007) Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J. Cell. Physiol. 213, 374–383 - PubMed
-
- Bailey J. M., Singh P. K., Hollingsworth M. A. (2007) Cancer metastasis facilitated by developmental pathways: sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell. Biochem. 102, 829–839 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

