Polarization dictates iron handling by inflammatory and alternatively activated macrophages
- PMID: 20511666
- PMCID: PMC2966902
- DOI: 10.3324/haematol.2010.023879
Polarization dictates iron handling by inflammatory and alternatively activated macrophages
Abstract
Background: Macrophages play a key role in iron homeostasis. In peripheral tissues, they are known to polarize into classically activated (or M1) macrophages and alternatively activated (or M2) macrophages. Little is known on whether the polarization program influences the ability of macrophages to store or recycle iron and the molecular machinery involved in the processes.
Design and methods: Inflammatory/M1 and alternatively activated/M2 macrophages were propagated in vitro from mouse bone-marrow precursors and polarized in the presence of recombinant interferon-γ or interleukin-4. We characterized and compared their ability to handle radioactive iron, the characteristics of the intracellular iron pools and the expression of molecules involved in internalization, storage and export of the metal. Moreover we verified the influence of iron on the relative ability of polarized macrophages to activate antigen-specific T cells.
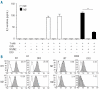
Results: M1 macrophages have low iron regulatory protein 1 and 2 binding activity, express high levels of ferritin H, low levels of transferrin receptor 1 and internalize--albeit with low efficiency -iron only when its extracellular concentration is high. In contrast, M2 macrophages have high iron regulatory protein binding activity, express low levels of ferritin H and high levels of transferrin receptor 1. M2 macrophages have a larger intracellular labile iron pool, effectively take up and spontaneously release iron at low concentrations and have limited storage ability. Iron export correlates with the expression of ferroportin, which is higher in M2 macrophages. M1 and M2 cells activate antigen-specific, MHC class II-restricted T cells. In the absence of the metal, only M1 macrophages are effective.
Conclusions: Cytokines that drive macrophage polarization ultimately control iron handling, leading to the differentiation of macrophages into a subset which has a relatively sealed intracellular iron content (M1) or into a subset endowed with the ability to recycle the metal (M2).
Figures


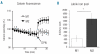


Comment in
-
Control of iron homeostasis as a key component of macrophage polarization.Haematologica. 2010 Nov;95(11):1801-3. doi: 10.3324/haematol.2010.030239. Haematologica. 2010. PMID: 21037324 Free PMC article. No abstract available.
References
-
- Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37(1):14–6. - PubMed
-
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. - PubMed
-
- De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9(1):72–81. - PubMed
-
- Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113(21):5277–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

