Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro
- PMID: 20511678
- PMCID: PMC2896872
- DOI: 10.1258/ebm.2010.009258
Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro
Abstract
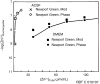
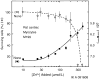
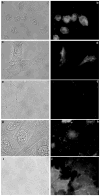
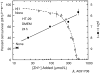
The zinc(II) ion has recently been implicated in a number of novel functions and pathologies in loci as diverse as the brain, retina, small intestine, prostate, heart, pancreas, and immune system. Zinc ions are a required nutrient but elevated concentrations are known to kill cells in vitro. Paradoxical observations regarding zinc's effects have appeared frequently in the literature, and often their physiological relevance is unclear. We found that for PC-12, HeLa and HT-29 cell lines as well as primary cultures of cardiac myocytes and neurons in vitro in differing media, approximately 5 nmol/L free zinc (pZn = 8.3, where pZn is defined as--log(10) [free Zn(2+)]) produced apparently healthy cells, but 20-fold higher or (in one case) lower concentrations were usually harmful as judged by multiple criteria. These results indicate that (1) the free zinc ion levels of media should be controlled with a metal ion buffer; (2) adding zinc or strong zinc ligands to an insufficiently buffered medium may lead to unpredictably low or high free zinc levels that are often harmful to cells; and (3) it is generally desirable to measure free zinc ion levels due to the presence of contaminating zinc in many biochemicals and unknown buffering capacity of many media.
Conflict of interest statement
Figures


References
-
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. - PubMed
-
- Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–5. - PubMed
-
- Maske H. Naturwissenschaften. 1955;42:424.
-
- Frederickson CJ, Klitenick MA, Manton WI, Kirkpatrick JB. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 1983;273:335–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

