Hypertonicity-induced mitochondrial membrane permeability in renal medullary interstitial cells: protective role of osmolytes
- PMID: 20511721
- PMCID: PMC3030460
- DOI: 10.1159/000315095
Hypertonicity-induced mitochondrial membrane permeability in renal medullary interstitial cells: protective role of osmolytes
Abstract
Background: Hyperosmotic stress causes cell death through activation of apoptotic pathways if the protective osmolyte response is impaired. In this study we attempt to elucidate the molecular mechanisms of hypertonicity-induced apoptosis and the effect of major organic osmolytes upon those.
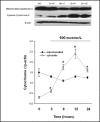
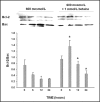
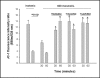
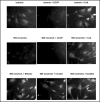
Methods: Hypertonicity-induced changes in Bcl2-family protein abundance and the presence of cytochrome c and apoptosis inducing factor (AIF) in the cytoplasm, were measured using western blot and immunofluorescence labeling. To determine dissipation of mitochondrial membrane potential (Delta Psi) though the permeability transition pore (PTP), the lipophilic cationic carbocyanine fluorescence probe JC-1 and TMRM fluorescence probes were used.
Results: Hypertonic culture conditions increase the abundance of proapoptotic Bax and the concentration of cytochrome c and apoptosis inducing factor (AIF) in the cytoplasm. These changes are associated with a dissipation of Delta Psi and increased permeability of the PTP. We further show that organic osmolytes stabilize the Delta Psi and decrease the concentration of cytochrome c and AIF in the cytoplasm.
Conclusion: Our study shows that organic osmolytes prevent hypertonicity-induced apoptosis by preventing dissipation of Delta Psi through stabilization of the PTP. These findings further support the important role of organic osmolytes in preventing hypertonicity-mediated cell death in medullary kidney cells.
Copyright 2010 S. Karger AG, Basel.
Figures


Similar articles
-
Hyperglycemia-induced oxidative stress exacerbates mitochondrial apoptosis damage to cochlear stria vascularis pericytes via the ROS-mediated Bcl-2/CytC/AIF pathway.Redox Rep. 2024 Dec;29(1):2382943. doi: 10.1080/13510002.2024.2382943. Epub 2024 Aug 2. Redox Rep. 2024. PMID: 39092597 Free PMC article.
-
Caspases 3 and 7: key mediators of mitochondrial events of apoptosis.Science. 2006 Feb 10;311(5762):847-51. doi: 10.1126/science.1115035. Science. 2006. PMID: 16469926 Free PMC article.
-
COX2 activity promotes organic osmolyte accumulation and adaptation of renal medullary interstitial cells to hypertonic stress.J Biol Chem. 2003 May 23;278(21):19352-7. doi: 10.1074/jbc.M302209200. Epub 2003 Mar 9. J Biol Chem. 2003. PMID: 12637551
-
The role of the mitochondrial permeability transition in cell death.Mitochondrion. 2006 Oct;6(5):225-34. doi: 10.1016/j.mito.2006.07.006. Epub 2006 Aug 1. Mitochondrion. 2006. PMID: 16935572 Review.
-
Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis.Anticancer Agents Med Chem. 2022;22(11):2048-2062. doi: 10.2174/1871520621666211126090906. Anticancer Agents Med Chem. 2022. PMID: 34825878 Review.
Cited by
-
Effects of low-dose bufalin combined with hydroxycamptothecin on human castration-resistant prostate cancer xenografts in nude mice.Exp Ther Med. 2021 Sep;22(3):1015. doi: 10.3892/etm.2021.10447. Epub 2021 Jul 15. Exp Ther Med. 2021. PMID: 34373701 Free PMC article.
-
High Concentration of Ketone Body β-Hydroxybutyrate Modifies Synaptic Vesicle Cycle and Depolarizes Plasma Membrane of Rat Brain Synaptosomes.J Mol Neurosci. 2020 Jan;70(1):112-119. doi: 10.1007/s12031-019-01406-9. Epub 2019 Oct 23. J Mol Neurosci. 2020. PMID: 31643037
-
Ionic imbalance, in addition to molecular crowding, abates cytoskeletal dynamics and vesicle motility during hypertonic stress.Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):E3104-13. doi: 10.1073/pnas.1421290112. Epub 2015 Jun 4. Proc Natl Acad Sci U S A. 2015. PMID: 26045497 Free PMC article.
-
Peptide affinity analysis of proteins that bind to an unstructured region containing the transactivating domain of the osmoprotective transcription factor NFAT5.Physiol Genomics. 2016 Nov 1;48(11):835-849. doi: 10.1152/physiolgenomics.00100.2016. Epub 2016 Oct 7. Physiol Genomics. 2016. PMID: 27764768 Free PMC article.
-
Peptide affinity analysis of proteins that bind to an unstructured NH2-terminal region of the osmoprotective transcription factor NFAT5.Physiol Genomics. 2016 Apr;48(4):290-305. doi: 10.1152/physiolgenomics.00110.2015. Epub 2016 Jan 12. Physiol Genomics. 2016. PMID: 26757802 Free PMC article.
References
-
- Moeckel GW, Zhang L, Fogo AB, Hao CM, Pozzi A, Breyer MD. COX2 activity promotes organic osmolyte accumulation and adaptation of renal medullary interstitial cells to hypertonic stress. J Biol Chem. 2003;278:19352–19357. - PubMed
-
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: Evolution of osmolyte system. Science. 1982;217:1214–1222. - PubMed
-
- Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiol Rev. 1991;71:1081–1115. - PubMed
-
- Kwon ED, Zablocki K, Jung KY, Peters EM, Garcia-Perez A, Burg MB. Osmoregulation of GPC:choline phosphaodiesterase in MDCK cells: different effects of urea and NaCl. Am J Physiol. 1995;269:C35–C41. - PubMed
-
- Neuhofer W, Beck FX. Cell Survival in the hostile environment of the renal medulla. Annu Rev Physiol. 2005;67:531–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
