Prp43p contains a processive helicase structural architecture with a specific regulatory domain
- PMID: 20512115
- PMCID: PMC2905241
- DOI: 10.1038/emboj.2010.102
Prp43p contains a processive helicase structural architecture with a specific regulatory domain
Abstract
The DEAH/RNA helicase A (RHA) helicase family comprises proteins involved in splicing, ribosome biogenesis and transcription regulation. We report the structure of yeast Prp43p, a DEAH/RHA helicase remarkable in that it functions in both splicing and ribosome biogenesis. Prp43p displays a novel structural architecture with an unforeseen homology with the Ski2-like Hel308 DNA helicase. Together with the presence of a beta-hairpin in the second RecA-like domain, Prp43p contains all the structural elements of a processive helicase. Moreover, our structure reveals that the C-terminal domain contains an oligonucleotide/oligosaccharide-binding (OB)-fold placed at the entrance of the putative nucleic acid cavity. Deletion or mutations of this domain decrease the affinity of Prp43p for RNA and severely reduce Prp43p ATPase activity in the presence of RNA. We also show that this domain constitutes the binding site for the G-patch-containing domain of Pfa1p. We propose that the C-terminal domain, specific to DEAH/RHA helicases, is a central player in the regulation of helicase activity by binding both RNA and G-patch domain proteins.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




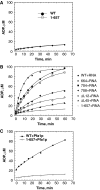

References
-
- Abrahams JP, Leslie AG (1996) Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr D Biol Crystallogr 52: 30–42 - PubMed
-
- Aravind L, Koonin EV (1999) G-patch: a new conserved domain in eukaryotic RNA-processing proteins and type D retroviral polyproteins. Trends Biochem Sci 24: 342–344 - PubMed
-
- Arcus V (2002) OB-fold domains: a snapshot of the evolution of sequence, structure and function. Curr Opin Struct Biol 12: 794–801 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

