Basophils and the T helper 2 environment can promote the development of lupus nephritis
- PMID: 20512127
- PMCID: PMC2909583
- DOI: 10.1038/nm.2159
Basophils and the T helper 2 environment can promote the development of lupus nephritis
Abstract
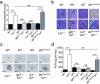
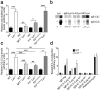
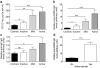
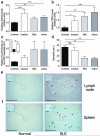
In systemic lupus erythematosus (SLE), self-reactive antibodies can target the kidney (lupus nephritis), leading to functional failure and possible mortality. We report that activation of basophils by autoreactive IgE causes their homing to lymph nodes, promoting T helper type 2 (T(H)2) cell differentiation and enhancing the production of self-reactive antibodies that cause lupus-like nephritis in mice lacking the Src family protein tyrosine kinase Lyn (Lyn(-/-) mice). Individuals with SLE also have elevated serum IgE, self-reactive IgEs and activated basophils that express CD62 ligand (CD62L) and the major histocompatibility complex (MHC) class II molecule human leukocyte antigen-DR (HLA-DR), parameters that are associated with increased disease activity and active lupus nephritis. Basophils were also present in the lymph nodes and spleen of subjects with SLE. Thus, in Lyn(-/-) mice, basophils and IgE autoantibodies amplify autoantibody production that leads to lupus nephritis, and in individuals with SLE IgE autoantibodies and activated basophils are factors associated with disease activity and nephritis.
Figures
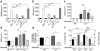
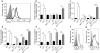
Comment in
-
Activated basophils give lupus a booster shot.Nat Med. 2010 Jun;16(6):635-6. doi: 10.1038/nm0610-635. Nat Med. 2010. PMID: 20526313 Free PMC article.
-
Autoimmunity: New players in lupus nephritis.Nat Rev Immunol. 2010 Jul;10(7):464. doi: 10.1038/nri2806. Nat Rev Immunol. 2010. PMID: 20586122 No abstract available.
-
Lupus nephritis: Activated basophils exacerbate lupus nephritis by amplifying production of autoreactive IgE.Nat Rev Rheumatol. 2010 Aug;6(8):438. doi: 10.1038/nrrheum.2010.111. Nat Rev Rheumatol. 2010. PMID: 20704010 No abstract available.
-
Basophils from humans with systemic lupus erythematosus do not express MHC-II.Nat Med. 2012 Apr 5;18(4):488-9; author reply 489-90. doi: 10.1038/nm.2663. Nat Med. 2012. PMID: 22481402 No abstract available.
Similar articles
-
Basophils and nephritis in lupus.N Engl J Med. 2010 Sep 9;363(11):1080-2. doi: 10.1056/NEJMcibr1006936. N Engl J Med. 2010. PMID: 20825323 No abstract available.
-
Basophils, IgE, and autoantibody-mediated kidney disease.J Immunol. 2011 Jun 1;186(11):6083-90. doi: 10.4049/jimmunol.1002648. J Immunol. 2011. PMID: 21597041 Free PMC article. Review.
-
Activated basophils give lupus a booster shot.Nat Med. 2010 Jun;16(6):635-6. doi: 10.1038/nm0610-635. Nat Med. 2010. PMID: 20526313 Free PMC article.
-
Basophils contribute to pristane-induced Lupus-like nephritis model.Sci Rep. 2017 Aug 11;7(1):7969. doi: 10.1038/s41598-017-08516-7. Sci Rep. 2017. PMID: 28801578 Free PMC article.
-
Basophils and autoreactive IgE in the pathogenesis of systemic lupus erythematosus.Curr Allergy Asthma Rep. 2011 Oct;11(5):378-87. doi: 10.1007/s11882-011-0216-5. Curr Allergy Asthma Rep. 2011. PMID: 21805094 Free PMC article. Review.
Cited by
-
Association of LncRNA-GAS5 gene polymorphisms and PBMC LncRNA-GAS5 level with risk of systemic lupus erythematosus in Chinese population.J Cell Mol Med. 2021 Apr;25(7):3548-3559. doi: 10.1111/jcmm.16438. Epub 2021 Mar 16. J Cell Mol Med. 2021. PMID: 33728802 Free PMC article.
-
Basophils from humans with systemic lupus erythematosus do not express MHC-II.Nat Med. 2012 Apr 5;18(4):488-9; author reply 489-90. doi: 10.1038/nm.2663. Nat Med. 2012. PMID: 22481402 No abstract available.
-
Role of basophils in rheumatoid arthritis (Review).Exp Ther Med. 2015 May;9(5):1567-1571. doi: 10.3892/etm.2015.2312. Epub 2015 Feb 24. Exp Ther Med. 2015. PMID: 26136859 Free PMC article.
-
Immunoprofiling of early, untreated rheumatoid arthritis using mass cytometry reveals an activated basophil subset inversely linked to ACPA status.Arthritis Res Ther. 2021 Oct 29;23(1):272. doi: 10.1186/s13075-021-02630-8. Arthritis Res Ther. 2021. PMID: 34715910 Free PMC article.
-
The skin is an important bulwark of acquired immunity against intestinal helminths.J Exp Med. 2013 Nov 18;210(12):2583-95. doi: 10.1084/jem.20130761. Epub 2013 Oct 28. J Exp Med. 2013. PMID: 24166714 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

