Uptake of carrier testing in families after cystic fibrosis diagnosis through newborn screening
- PMID: 20512163
- PMCID: PMC2987447
- DOI: 10.1038/ejhg.2010.78
Uptake of carrier testing in families after cystic fibrosis diagnosis through newborn screening
Abstract
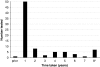
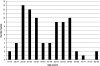
Newborn screening (NBS) for cystic fibrosis (CF) provides the opportunity for cascade carrier testing of relatives. Uptake of testing by adult non-parent relatives of children diagnosed with CF through NBS has not been previously described, and this study describes uptake by both parents and adult non-parent relatives in Victoria, Australia. Pedigrees were taken from parents of children who were born in 2000-2004 and diagnosed with CF. A total of 40 families were eligible for the study and 30 (75%) were recruited. In all, 716 non-parent relatives were identified from the pedigrees as eligible for carrier testing, and 82 (adjusted uptake percentage: 11.8%; 95% confidence interval 8.0-15.7) have had carrier testing by March 2009. On average, 2.7 non-parent relatives per family had CF carrier testing after diagnosis through NBS. The odds of being tested were greater for females than males (adjusted odds ratio 1.61; 95% confidence interval 1.11-2.33; P=0.01) and greater for those more closely related to the child with CF (adjusted odds ratio 5.17; 95% confidence interval 2.38-11.24; P<0.001). Most relatives who undergo testing are tested immediately after the baby's diagnosis; however, some testing is undertaken up to 8 years later. These results indicate that in a clinical setting, the diagnosis of a baby with CF by NBS does not lead to carrier testing for the majority of the baby's non-parent relatives. We suggest re-contact with parents to offer cascade carrier testing.
Figures
Similar articles
-
Community-wide screening for cystic fibrosis carriers could replace newborn screening for the diagnosis of cystic fibrosis.J Paediatr Child Health. 2007 Nov;43(11):721-3. doi: 10.1111/j.1440-1754.2007.01224.x. J Paediatr Child Health. 2007. PMID: 17924936
-
Genetic counseling and neonatal screening for cystic fibrosis: an assessment of the communication process.Pediatrics. 2001 Apr;107(4):699-705. doi: 10.1542/peds.107.4.699. Pediatrics. 2001. PMID: 11335747
-
Improvement in cystic fibrosis newborn screening program outcomes with genetic counseling via telemedicine.Pediatr Pulmonol. 2023 Dec;58(12):3478-3486. doi: 10.1002/ppul.26678. Epub 2023 Sep 15. Pediatr Pulmonol. 2023. PMID: 37712603
-
Patient and family issues regarding genetic testing for cystic fibrosis: a review of prenatal carrier testing and newborn screening.Annu Rev Nurs Res. 2011;29:303-29. doi: 10.1891/0739-6686.29.303. Annu Rev Nurs Res. 2011. PMID: 22891510 Review.
-
Cystic Fibrosis Diagnosis in Newborns, Children, and Adults.Semin Respir Crit Care Med. 2019 Dec;40(6):701-714. doi: 10.1055/s-0039-1697961. Epub 2019 Nov 3. Semin Respir Crit Care Med. 2019. PMID: 31679154 Review.
Cited by
-
The Role of Extended CFTR Gene Sequencing in Newborn Screening for Cystic Fibrosis.Int J Neonatal Screen. 2020 Mar 21;6(1):23. doi: 10.3390/ijns6010023. eCollection 2020 Mar. Int J Neonatal Screen. 2020. PMID: 33073020 Free PMC article. Review.
-
Cascade health service use in family members following genetic testing in children: a scoping literature review.Eur J Hum Genet. 2021 Nov;29(11):1601-1610. doi: 10.1038/s41431-021-00952-4. Epub 2021 Aug 26. Eur J Hum Genet. 2021. PMID: 34446836 Free PMC article.
-
Psychosocial Distress and Knowledge Deficiencies in Parents of Children in Ireland Who Carry an Altered Cystic Fibrosis Gene.J Genet Couns. 2018 Jun;27(3):589-596. doi: 10.1007/s10897-017-0150-3. Epub 2017 Sep 26. J Genet Couns. 2018. PMID: 28952009 Clinical Trial.
-
Carrier screening in preconception consultation in primary care.J Community Genet. 2012 Jul;3(3):193-203. doi: 10.1007/s12687-011-0071-z. Epub 2011 Dec 20. J Community Genet. 2012. PMID: 22183783 Free PMC article.
-
Attitudes and opinions of pregnant women who are not offered cystic fibrosis carrier screening.Eur J Hum Genet. 2014 Jul;22(7):859-65. doi: 10.1038/ejhg.2013.267. Epub 2013 Nov 20. Eur J Hum Genet. 2014. PMID: 24253861 Free PMC article. Clinical Trial.
References
-
- Southern KW, Munck A, Pollitt R, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6:57–65. - PubMed
-
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
-
- National Institutes of Health Consensus development conference statement on genetic testing for cystic fibrosis. Arch Intern Med. 1999;159:1529–1539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

