Specific immunotherapy using Hymenoptera venom: systematic review
- PMID: 20512278
- PMCID: PMC10936127
- DOI: 10.1590/s1516-31802010000100007
Specific immunotherapy using Hymenoptera venom: systematic review
Abstract
Context and objective: The only effective treatment for patients who have severe reactions after Hymenoptera stings is venom immunotherapy. The aim of this study was to review the literature to assess the effects of venom immunotherapy among patients presenting severe reactions after Hymenoptera stings.
Design and setting: Randomized controlled trials in the worldwide literature were reviewed. The manuscript was produced in the Discipline of Allergy and Clinical Immunology, Universidade de São Paulo (USP).
Methods: Randomized controlled trials involving venom immunotherapy versus placebo or only patient follow-up were evaluated. The risk of systemic reactions after specific immunotherapy was evaluated by calculating odds ratios (OR) and their 95% confidence intervals.
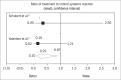
Results: 2,273 abstracts were identified by the keywords search. Only four studies were included in this review. The chi-square test for heterogeneity showed that two studies were homogeneous and could be included in a meta-analysis. By combining the two studies, the odds ratio became significant: 0.29 (0.10-0.87). However, analysis on the severity of the reactions after immunotherapy showed that the benefits may not be so significant because the reactions were mostly similar to or milder than the original reaction.
Conclusions: Specific immunotherapy should be recommended for adults and children with moderate to severe reactions, but there is no need to prescribe it for children with skin reactions alone, especially if the exposure is very sporadic. On the other hand, the risk-benefit relation should always be assessed in each case.
CONTEXTO E OBJETIVO:: O único tratamento eficaz para pacientes que têm reações graves após ferroada de Hymenoptera é a imunoterapia com veneno. O objetivo deste estudo foi rever a literatura para avaliar os efeitos da imunoterapia com veneno em pacientes com reações graves após ferroada de Hymenoptera.
TIPO DE ESTUDO E LOCAL:: Foram revisados na literatura mundial ensaios clínicos controlados e aleatórios. O manuscrito foi realizado na Disciplina de Alergia e Imunologia Clínica, Universidade de São Paulo (USP).
Métodos:: Ensaios clínicos controlados e aleatórios envolvendo imunoterapia com veneno de Hymenoptera versus placebo ou apenas acompanhamento dos pacientes foram avaliados. Realizada imunoterapia específica, o risco de reações sistêmicas foi avaliado através de cálculo do “odds ratio” e intervalo de confiança de 95%.
RESULTADOS:: 2.273 resumos foram identificados na busca pelas palavras chave. Apenas quatro estudos foram incluídos nesta revisão. O teste qui-quadrado de heterogeneidade mostrou que dois estudos foram homogêneos e puderam ser incluídos na metanálise. Ao combinar os dois estudos, o “odds ratio” passou a ser significativo: 0.29 (0.10-0.87). Entretanto, ao analisar a gravidade das reações ocorridas após a imunoterapia, observou-se que os benefícios podem não ser tão relevantes, pois as reações foram, na grande maioria, ou mais leves ou semelhantes à reação original.
CONCLUSÕES:: A imunoterapia específica deve ser recomendada para adultos e crianças com reações moderadas a graves, porém não há necessidade de prescrevê-la para as crianças apenas com reações cutâneas, especialmente se a exposição é muito esporádica. No outro lado, a relação risco-benefício deve ser sempre avaliada em cada caso.
Conflict of interest statement
Figures
References
-
- Helbling A, Hurni T, Mueller UR, Pitchler WJ. Incidence of anaphylaxis with circulatory symptoms: a study over a 3-year period comprising 940,000 inhabitants of the Swiss Canton Bern. Clin Exp Allergy. 2004;34(2):285–290. - PubMed
-
- Golden DB, Marsh DG, Kagey-Sobotka A, et al. Epidemiology of insect venom sensitivity. JAMA. 1989;262(2):240–244. - PubMed
-
- Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115(3):439–447. quiz 448. - PubMed
-
- Incorvaia C, Pucci S, Pastorello EA. Clinical aspects of Hymenoptera venom allergy. Allergy. 1999;54(Suppl 58):50–52. - PubMed
-
- The discontinuation of Hymenoptera venom immunotherapy Report from the Committee on Insects. J Allergy Clin Immunol. 1998;101(5):573–575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

