Saliency, switching, attention and control: a network model of insula function
- PMID: 20512370
- PMCID: PMC2899886
- DOI: 10.1007/s00429-010-0262-0
Saliency, switching, attention and control: a network model of insula function
Abstract
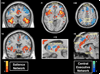
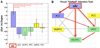
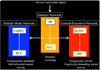
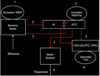
The insula is a brain structure implicated in disparate cognitive, affective, and regulatory functions, including interoceptive awareness, emotional responses, and empathic processes. While classically considered a limbic region, recent evidence from network analysis suggests a critical role for the insula, particularly the anterior division, in high-level cognitive control and attentional processes. The crucial insight and view we present here is of the anterior insula as an integral hub in mediating dynamic interactions between other large-scale brain networks involved in externally oriented attention and internally oriented or self-related cognition. The model we present postulates that the insula is sensitive to salient events, and that its core function is to mark such events for additional processing and initiate appropriate control signals. The anterior insula and the anterior cingulate cortex form a "salience network" that functions to segregate the most relevant among internal and extrapersonal stimuli in order to guide behavior. Within the framework of our network model, the disparate functions ascribed to the insula can be conceptualized by a few basic mechanisms: (1) bottom-up detection of salient events, (2) switching between other large-scale networks to facilitate access to attention and working memory resources when a salient event is detected, (3) interaction of the anterior and posterior insula to modulate autonomic reactivity to salient stimuli, and (4) strong functional coupling with the anterior cingulate cortex that facilitates rapid access to the motor system. In this manner, with the insula as its integral hub, the salience network assists target brain regions in the generation of appropriate behavioral responses to salient stimuli. We suggest that this framework provides a parsimonious account of insula function in neurotypical adults, and may provide novel insights into the neural basis of disorders of affective and social cognition.
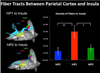
Figures

Similar articles
-
The anterior insula in autism: under-connected and under-examined.Neurosci Biobehav Rev. 2009 Sep;33(8):1198-203. doi: 10.1016/j.neubiorev.2009.06.002. Epub 2009 Jun 16. Neurosci Biobehav Rev. 2009. PMID: 19538989 Free PMC article. Review.
-
Functional Connectivity of Insula, Basal Ganglia, and Prefrontal Executive Control Networks during Hypoglycemia in Type 1 Diabetes.J Neurosci. 2015 Aug 5;35(31):11012-23. doi: 10.1523/JNEUROSCI.0319-15.2015. J Neurosci. 2015. PMID: 26245963 Free PMC article.
-
Functional connectivity of the insula in the resting brain.Neuroimage. 2011 Mar 1;55(1):8-23. doi: 10.1016/j.neuroimage.2010.11.049. Epub 2010 Nov 24. Neuroimage. 2011. PMID: 21111053
-
Anterior insula as a gatekeeper of executive control.Neurosci Biobehav Rev. 2022 Aug;139:104736. doi: 10.1016/j.neubiorev.2022.104736. Epub 2022 Jun 11. Neurosci Biobehav Rev. 2022. PMID: 35700753 Review.
-
Functional brain basis of hypnotizability.Arch Gen Psychiatry. 2012 Oct;69(10):1064-72. doi: 10.1001/archgenpsychiatry.2011.2190. Arch Gen Psychiatry. 2012. PMID: 23026956 Free PMC article.
Cited by
-
Diffusion and functional MRI reveal microstructural and network connectivity impairment in adult-onset neuronal intranuclear inclusion disease.Front Aging Neurosci. 2024 Oct 11;16:1478065. doi: 10.3389/fnagi.2024.1478065. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39463819 Free PMC article.
-
Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury.Dev Cogn Neurosci. 2013 Jul;5:25-39. doi: 10.1016/j.dcn.2012.11.006. Epub 2012 Nov 23. Dev Cogn Neurosci. 2013. PMID: 23291312 Free PMC article.
-
Stimulation in the Rat Anterior Insula and Anterior Cingulate During an Effortful Weightlifting Task.Front Neurosci. 2021 Feb 26;15:643384. doi: 10.3389/fnins.2021.643384. eCollection 2021. Front Neurosci. 2021. PMID: 33716659 Free PMC article.
-
The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network.Pain. 2015 Sep;156(9):1755-1764. doi: 10.1097/j.pain.0000000000000238. Pain. 2015. PMID: 26010458 Free PMC article.
-
Disease-related differences in resting-state networks: a comparison between localized provoked vulvodynia, irritable bowel syndrome, and healthy control subjects.Pain. 2015 May;156(5):809-819. doi: 10.1097/01.j.pain.0000461289.65571.54. Pain. 2015. PMID: 25735001 Free PMC article.
References
-
- Afif A, Mertens P. Description of sulcal organization of the insular cortex. Surg Radiol Anat. 2010 (in press) - PubMed
-
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9(8):367–373. - PubMed
-
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214(5–6) doi: 10.1007/s00429-010-0254-0. - DOI - PubMed
-
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. - PubMed
-
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

