Neurod1 regulates survival and formation of connections in mouse ear and brain
- PMID: 20512592
- PMCID: PMC3657738
- DOI: 10.1007/s00441-010-0984-6
Neurod1 regulates survival and formation of connections in mouse ear and brain
Abstract
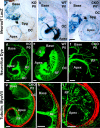
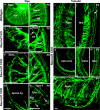
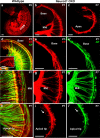
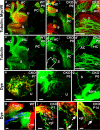
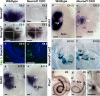
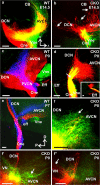
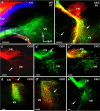
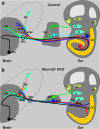
The developing sensory neurons of the mammalian ear require two sequentially activated bHLH genes, Neurog1 and Neurod1, for their development. Neurons never develop in Neurog1 null mice, and most neurons die in Neurod1 null mutants, a gene upregulated by Neurog1. The surviving neurons of Neurod1 null mice are incompletely characterized in postnatal mice because of the early lethality of mutants and the possible compromising effect of the absence of insulin on peripheral neuropathies. Using Tg(Pax2-cre), we have generated a conditional deletion of floxed Neurod1 for the ear; this mouse is viable and allows us to investigate ear innervation defects of Neurod1 absence only in the ear. We have compared the defects in embryos and show an ear phenotype in conditional Neurod1 null mice comparable with the systemic Neurod1 null mouse. By studying postnatal animals, we show that Neurod1 not only is necessary for the survival of most spiral and many vestibular neurons, but is also essential for a segregated central projection of vestibular and cochlear afferents. In the absence of Neurod1 in the ear, vestibular and cochlear afferents enter the cochlear nucleus as a single mixed nerve. Neurites coming from vestibular and cochlear sensory epithelia project centrally to both cochlear and vestibular nuclei, in addition to their designated target projections. The peripheral innervation of the remaining sensory neurons is disorganized and shows collaterals of single neurons projecting to multiple endorgans, displaying no tonotopic organization of the organ of Corti or the cochlear nucleus. Pending elucidation of the molecular details for these Neurod1 functions, these data demonstrate that Neurod1 is not only a major factor for the survival of neurons but is crucial for the development of normal ear connections, both in the ear and in the central nervous system.
Figures

Similar articles
-
Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea.PLoS One. 2010 Jul 22;5(7):e11661. doi: 10.1371/journal.pone.0011661. PLoS One. 2010. PMID: 20661473 Free PMC article.
-
Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation.BMC Dev Biol. 2010 Aug 20;10:89. doi: 10.1186/1471-213X-10-89. BMC Dev Biol. 2010. PMID: 20727173 Free PMC article.
-
Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems.Genes Dev. 2000 Nov 15;14(22):2839-54. doi: 10.1101/gad.840500. Genes Dev. 2000. PMID: 11090132 Free PMC article.
-
Development in the Mammalian Auditory System Depends on Transcription Factors.Int J Mol Sci. 2021 Apr 18;22(8):4189. doi: 10.3390/ijms22084189. Int J Mol Sci. 2021. PMID: 33919542 Free PMC article. Review.
-
Neurog1, Neurod1, and Atoh1 are essential for spiral ganglia, cochlear nuclei, and cochlear hair cell development.Fac Rev. 2021 May 11;10:47. doi: 10.12703/r/10-47. eCollection 2021. Fac Rev. 2021. PMID: 34131657 Free PMC article. Review.
Cited by
-
Transcriptomic Analysis Identifies Candidate Genes for Differential Expression during Xenopus laevis Inner Ear Development.bioRxiv [Preprint]. 2024 Jan 1:2023.12.29.573599. doi: 10.1101/2023.12.29.573599. bioRxiv. 2024. PMID: 38260420 Free PMC article. Preprint.
-
Npr2 null mutants show initial overshooting followed by reduction of spiral ganglion axon projections combined with near-normal cochleotopic projection.Cell Tissue Res. 2019 Oct;378(1):15-32. doi: 10.1007/s00441-019-03050-6. Epub 2019 Jun 14. Cell Tissue Res. 2019. PMID: 31201541 Free PMC article.
-
An Integrated Perspective of Evolution and Development: From Genes to Function to Ear, Lateral Line and Electroreception.Diversity (Basel). 2021 Aug;13(8):364. doi: 10.3390/d13080364. Epub 2021 Aug 7. Diversity (Basel). 2021. PMID: 35505776 Free PMC article.
-
Selective tracing of auditory fibers in the avian embryonic vestibulocochlear nerve.J Vis Exp. 2013 Mar 18;(73):e50305. doi: 10.3791/50305. J Vis Exp. 2013. PMID: 23542875 Free PMC article.
-
Sensory afferent segregation in three-eared frogs resemble the dominance columns observed in three-eyed frogs.Sci Rep. 2015 Feb 9;5:8338. doi: 10.1038/srep08338. Sci Rep. 2015. PMID: 25661240 Free PMC article.
References
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. - PubMed
-
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

