Triple repetition time saturation transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics
- PMID: 20512852
- PMCID: PMC2926802
- DOI: 10.1002/mrm.22347
Triple repetition time saturation transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics
Abstract
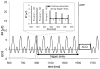
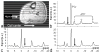
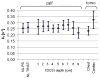
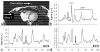
Human cardiac phosphorus MR saturation transfer experiments to quantify creatine kinase forward rate constants (k(f)) have previously been performed at 1.5 T. Such experiments could benefit from increased signal-to-noise ratio (SNR) and spectral resolution at 3 T. At 1.5 T, the four-angle saturation transfer method was applied with low-angle adiabatic pulses and surface coils. However, low-angle adiabatic pulses are potentially problematic above 1.5 T due to bandwidth limitations, power requirements, power deposition, and intrapulse spin-spin relaxation. For localized metabolite spin-lattice relaxation time (T(1)) measurements, a dual repetition time approach with adiabatic half-passage pulses was recently introduced to solve these problems at 3 T. Because the saturation transfer experiment requires a T(1) measurement performed while one reacting moiety is saturated, we adapt the dual repetition time approach to measure k(f) using a triple repetition time saturation transfer (TRiST) method. A new pulsed saturation scheme with reduced sensitivity to static magnetic field inhomogeneity and compatibility with cardiac triggering is also presented. TRiST measurements of k(f) are validated in human calf muscle against conventional saturation transfer and found to agree within 3%. The first 3-T TRiST measurements of creatine kinase k(f) in the human calf (n = 6), chest muscle, and heart (n = 8) are 0.26 +/- 0.04 s(-1), 0.23 +/- 0.03 s(-1), and 0.32 +/- 0.07 s(-1), respectively, consistent with prior 1.5 T values.
(c) 2010 Wiley-Liss, Inc.
Figures



Similar articles
-
Two repetition time saturation transfer (TwiST) with spill-over correction to measure creatine kinase reaction rates in human hearts.J Cardiovasc Magn Reson. 2015 Aug 8;17(1):70. doi: 10.1186/s12968-015-0175-4. J Cardiovasc Magn Reson. 2015. PMID: 26253320 Free PMC article.
-
Localized rest and stress human cardiac creatine kinase reaction kinetics at 3 T.NMR Biomed. 2019 Jun;32(6):e4085. doi: 10.1002/nbm.4085. Epub 2019 Mar 28. NMR Biomed. 2019. PMID: 30920054 Free PMC article.
-
Quantitative cardiac 31P spectroscopy at 3 Tesla using adiabatic pulses.Magn Reson Med. 2009 Apr;61(4):785-95. doi: 10.1002/mrm.21867. Magn Reson Med. 2009. PMID: 19195018 Free PMC article.
-
Comparison of (31)P saturation and inversion magnetization transfer in human liver and skeletal muscle using a clinical MR system and surface coils.NMR Biomed. 2015 Feb;28(2):188-99. doi: 10.1002/nbm.3242. Epub 2014 Dec 7. NMR Biomed. 2015. PMID: 25483778
-
Four-angle saturation transfer (FAST) method for measuring creatine kinase reaction rates in vivo.Magn Reson Med. 2002 May;47(5):850-63. doi: 10.1002/mrm.10130. Magn Reson Med. 2002. PMID: 11979563 Free PMC article.
Cited by
-
Cardiac work is related to creatine kinase energy supply in human heart failure: a cardiovascular magnetic resonance spectroscopy study.J Cardiovasc Magn Reson. 2018 Dec 10;20(1):81. doi: 10.1186/s12968-018-0491-6. J Cardiovasc Magn Reson. 2018. PMID: 30526611 Free PMC article.
-
Quantification of human high-energy phosphate metabolite concentrations at 3 T with partial volume and sensitivity corrections.NMR Biomed. 2013 Nov;26(11):1363-71. doi: 10.1002/nbm.2961. Epub 2013 Jun 3. NMR Biomed. 2013. PMID: 23729378 Free PMC article.
-
Creatine kinase rate constant in the human heart at 7T with 1D-ISIS/2D CSI localization.PLoS One. 2020 Mar 19;15(3):e0229933. doi: 10.1371/journal.pone.0229933. eCollection 2020. PLoS One. 2020. PMID: 32191723 Free PMC article.
-
OXSA: An open-source magnetic resonance spectroscopy analysis toolbox in MATLAB.PLoS One. 2017 Sep 22;12(9):e0185356. doi: 10.1371/journal.pone.0185356. eCollection 2017. PLoS One. 2017. PMID: 28938003 Free PMC article.
-
High-energy phosphate transfer in human muscle: diffusion of phosphocreatine.Am J Physiol Cell Physiol. 2011 Jul;301(1):C234-41. doi: 10.1152/ajpcell.00500.2010. Epub 2011 Mar 2. Am J Physiol Cell Physiol. 2011. PMID: 21368292 Free PMC article.
References
-
- Forsen S, Hoffman RA. Study of Moderately Rapid Chemical Exchange Reactions by Means of Nuclear Magnetic Double Resonance. Journal of Chemical Physics. 1963;39(11):2892.
-
- Bottomley PA, Hardy CJ. Mapping Creatine-Kinase Reaction-Rates in Human Brain and Heart with 4-Tesla Saturation Transfer P-31 Nmr. Journal of Magnetic Resonance. 1992;99(2):443–448.
-
- Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–114. - PubMed
-
- Horska A, Fishbein KW, Fleg JL, Spencer RG. The relationship between creatine kinase kinetics and exercise intensity in human forearm is unchanged by age. American journal of physiology. 2000;279(2):E333–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

