Beta-catenin--a supporting role in the skeleton
- PMID: 20512915
- PMCID: PMC3750230
- DOI: 10.1002/jcb.22574
Beta-catenin--a supporting role in the skeleton
Abstract
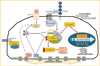
In the last 5 years a role for beta-catenin in the skeleton has been cemented. Beginning with mutations in the Lrp5 receptor that control beta-catenin canonical downstream signals, and progressing to transgenic models with bone-specific alteration of beta-catenin, research has shown that beta-catenin is required for normal bone development. A cell critical to bone in which beta-catenin activity determines function is the marrow-derived mesenchymal stem cell (MSC), where sustained beta-catenin prevents its distribution into adipogenic lineage. beta-Catenin actions are less well understood in mature osteoblasts: while beta-catenin contributes to control of osteoclastic bone resorption via alteration of the osteoprotegerin/RANKL ratio, a specific regulatory role during osteoblast bone synthesis has not yet been determined. The proven ability of mechanical factors to prevent beta-catenin degradation and induce nuclear translocation through Lrp-independent mechanisms suggests processes by which exercise might modulate bone mass via control of lineage allocation, in particular, by preventing precursor distribution into the adipocyte pool. Effects resulting from mechanical activation of beta-catenin in mature osteoblasts and osteocytes likely modulate bone resorption, but whether beta-catenin is involved in osteoblast synthetic function remains to be proven for both mechanical and soluble mediators. As beta-catenin appears to support the downstream effects of multiple osteogenic factors, studies clarifying when and where beta-catenin effects occur will be relevant for translational approaches aimed at preventing bone loss and terminal adipogenic conversion.
(c) 2010 Wiley-Liss, Inc.
Figures



References
-
- Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/beta-catenin signaling is a component of osteoblastic bone cells’ early responses to load-bearing, and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. - PubMed
-
- Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. - PubMed
-
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. - PubMed
-
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

