The use of native cation-exchange chromatography to study aggregation and phase separation of monoclonal antibodies
- PMID: 20512972
- PMCID: PMC2895243
- DOI: 10.1002/pro.396
The use of native cation-exchange chromatography to study aggregation and phase separation of monoclonal antibodies
Abstract
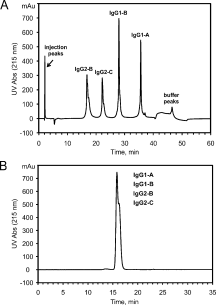
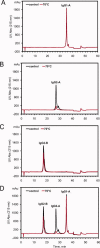
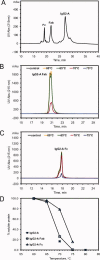
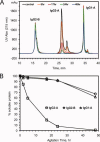
This study introduces a novel analytical approach for studying aggregation and phase separation of monoclonal antibodies (mAbs). The approach is based on using analytical scale cation-exchange chromatography (CEX) for measuring the loss of soluble monomer in the case of individual and mixed protein solutions. Native CEX outperforms traditional size-exclusion chromatography in separating complex protein mixtures, offering an easy way to assess mAb aggregation propensity. Different IgG1 and IgG2 molecules were tested individually and in mixtures consisting of up to four protein molecules. Antibody aggregation was induced by four different stress factors: high temperature, low pH, addition of fatty acids, and rigorous agitation. The extent of aggregation was determined from the amount of monomeric protein remaining in solution after stress. Consequently, it was possible to address the role of specific mAb regions in antibody aggregation by co-incubating Fab and Fc fragments with their respective full-length molecules. Our results revealed that the relative contribution of Fab and Fc regions in mAb aggregation is strongly dependent on pH and the stress factor applied. In addition, the CEX-based approach was used to study reversible protein precipitation due to phase separation, which demonstrated its use for a broader range of protein-protein association phenomena. In all cases, the role of Fab and Fc was clearly dissected, providing important information for engineering more stable mAb-based therapeutics.
Figures



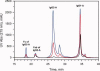
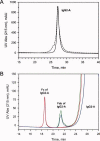
References
-
- Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10:307–377. - PubMed
-
- Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289:1–30. - PubMed
-
- Weiss WF, IV, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98:1246–1277. - PubMed
-
- Roberts CJ. Non-native protein aggregation: pathways, kinetics, and shelf-life prediction. In: Murphy RM, Tsai A, editors. Misbehaving proteins: protein (mis)folding, aggregation, and stability. New York: Springer; 2006. pp. 17–46.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

