Multiple effects of silymarin on the hepatitis C virus lifecycle
- PMID: 20512985
- PMCID: PMC2909978
- DOI: 10.1002/hep.23587
Multiple effects of silymarin on the hepatitis C virus lifecycle
Abstract
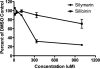
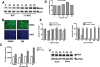
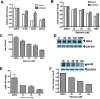
Silymarin, an extract from milk thistle (Silybum marianum), and its purified flavonolignans have been recently shown to inhibit hepatitis C virus (HCV) infection, both in vitro and in vivo. In the current study, we further characterized silymarin's antiviral actions. Silymarin had antiviral effects against hepatitis C virus cell culture (HCVcc) infection that included inhibition of virus entry, RNA and protein expression, and infectious virus production. Silymarin did not block HCVcc binding to cells but inhibited the entry of several viral pseudoparticles (pp), and fusion of HCVpp with liposomes. Silymarin but not silibinin inhibited genotype 2a NS5B RNA-dependent RNA polymerase (RdRp) activity at concentrations 5 to 10 times higher than required for anti-HCVcc effects. Furthermore, silymarin had inefficient activity on the genotype 1b BK and four 1b RDRPs derived from HCV-infected patients. Moreover, silymarin did not inhibit HCV replication in five independent genotype 1a, 1b, and 2a replicon cell lines that did not produce infectious virus. Silymarin inhibited microsomal triglyceride transfer protein activity, apolipoprotein B secretion, and infectious virion production into culture supernatants. Silymarin also blocked cell-to-cell spread of virus.
Conclusion: Although inhibition of in vitro NS5B polymerase activity is demonstrable, the mechanisms of silymarin's antiviral action appear to include blocking of virus entry and transmission, possibly by targeting the host cell.
Figures
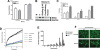
Comment in
-
Silibinin mode of action against hepatitis C virus: a controversy yet to be resolved.Hepatology. 2011 Aug;54(2):749. doi: 10.1002/hep.24310. Epub 2011 Jun 27. Hepatology. 2011. PMID: 21433039 Free PMC article. No abstract available.
References
-
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. - PubMed
-
- Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, et al. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605–612. - PubMed
-
- Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. - PubMed
-
- Rainone F. Milk thistle. Am Fam Physician. 2005;72:1285–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R21 AT002895/AT/NCCIH NIH HHS/United States
- R01 CA104286/CA/NCI NIH HHS/United States
- G0400802/MRC_/Medical Research Council/United Kingdom
- P30-DK040561/DK/NIDDK NIH HHS/United States
- CA104286/CA/NCI NIH HHS/United States
- AI-25488/AI/NIAID NIH HHS/United States
- N01 AI040034/AI/NIAID NIH HHS/United States
- AT002895/AT/NCCIH NIH HHS/United States
- G0801976/MRC_/Medical Research Council/United Kingdom
- R21 CA125321/CA/NCI NIH HHS/United States
- R01 AI050798/AI/NIAID NIH HHS/United States
- AI40034-14/AI/NIAID NIH HHS/United States
- K01-DK080241/DK/NIDDK NIH HHS/United States
- K01 DK080241/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- AI50798/AI/NIAID NIH HHS/United States
- N01 AI025488/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
