CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium
- PMID: 20512991
- PMCID: PMC2919204
- DOI: 10.1002/hep.23591
CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium
Abstract
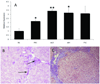
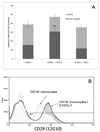
The liver contains macrophages and myeloid dendritic cells (mDCs) that are critical for the regulation of hepatic inflammation. Most hepatic macrophages and mDCs are derived from monocytes recruited from the blood through poorly understood interactions with hepatic sinusoidal endothelial cells (HSECs). Human CD16(+) monocytes are thought to contain the precursor populations for tissue macrophages and mDCs. We report that CD16(+) cells localize to areas of active inflammation and fibrosis in chronic inflammatory liver disease and that a unique combination of cell surface receptors promotes the transendothelial migration of CD16(+) monocytes through human HSECs under physiological flow. CX(3)CR1 activation was the dominant pertussis-sensitive mechanism controlling transendothelial migration under flow, and expression of the CX(3)CR1 ligand CX(3)CL1 is increased on hepatic sinusoids in chronic inflammatory liver disease. Exposure of CD16(+) monocytes to immobilized purified CX(3)CL1 triggered beta1-integrin-mediated adhesion to vascular cell adhesion molecule-1 and induced the development of a migratory phenotype. Following transmigration or exposure to soluble CX(3)CL1, CD16(+) monocytes rapidly but transiently lost expression of CX(3)CR1. Adhesion and transmigration across HSECs under flow was also dependent on vascular adhesion protein-1 (VAP-1) on the HSECs.
Conclusion: Our data suggest that CD16(+) monocytes are recruited by a combination of adhesive signals involving VAP-1 and CX(3)CR1 mediated integrin-activation. Thus a novel combination of surface molecules, including VAP-1 and CX(3)CL1 promotes the recruitment of CD16(+) monocytes to the liver, allowing them to localize at sites of chronic inflammation and fibrosis.
Figures





Similar articles
-
The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome.J Hepatol. 2012 Oct;57(4):752-8. doi: 10.1016/j.jhep.2012.05.014. Epub 2012 May 29. J Hepatol. 2012. PMID: 22659346 Free PMC article.
-
Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions.Immunobiology. 2004;209(1-2):11-20. doi: 10.1016/j.imbio.2004.04.001. Immunobiology. 2004. PMID: 15481136
-
Human CD16+ monocytes promote a pro-atherosclerotic endothelial cell phenotype via CX3CR1-CX3CL1 interaction.Cardiovasc Res. 2021 May 25;117(6):1510-1522. doi: 10.1093/cvr/cvaa234. Cardiovasc Res. 2021. PMID: 32717023
-
CX3CL1/fractalkine is a novel regulator of normal and malignant human B cell function.J Leukoc Biol. 2012 Jul;92(1):51-8. doi: 10.1189/jlb.0112035. Epub 2012 Mar 27. J Leukoc Biol. 2012. PMID: 22457367 Review.
-
Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo.World J Gastroenterol. 2006 Sep 14;12(34):5429-39. doi: 10.3748/wjg.v12.i34.5429. World J Gastroenterol. 2006. PMID: 17006978 Free PMC article. Review.
Cited by
-
Molecular mechanisms of pancreatic cancer liver metastasis: the role of PAK2.Front Immunol. 2024 Jan 26;15:1347683. doi: 10.3389/fimmu.2024.1347683. eCollection 2024. Front Immunol. 2024. PMID: 38343537 Free PMC article.
-
Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B.PLoS One. 2011 Mar 1;6(3):e17484. doi: 10.1371/journal.pone.0017484. PLoS One. 2011. PMID: 21390263 Free PMC article.
-
Inhibition of vascular adhesion protein-1 modifies hepatic steatosis in vitro and in vivo.World J Hepatol. 2020 Nov 27;12(11):931-948. doi: 10.4254/wjh.v12.i11.931. World J Hepatol. 2020. PMID: 33312420 Free PMC article.
-
Inhibition of vascular adhesion protein-1 enhances the anti-tumor effects of immune checkpoint inhibitors.Cancer Sci. 2021 Apr;112(4):1390-1401. doi: 10.1111/cas.14812. Epub 2021 Mar 18. Cancer Sci. 2021. PMID: 33453147 Free PMC article.
-
Innate immune cells in liver inflammation.Mediators Inflamm. 2012;2012:949157. doi: 10.1155/2012/949157. Epub 2012 Aug 9. Mediators Inflamm. 2012. PMID: 22933833 Free PMC article. Review.
References
-
- Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, Kikuchi K, Ikehara S, Gershwin ME. Heterogeneity of dendritic cells in the mouse liver. J Immunol. 2003;170:2323–2330. - PubMed
-
- Abe M, Zahorchak AF, Colvin BL, Thomson AW. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation. 2004;78:762–765. - PubMed
-
- Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
