Membrane bending energy and fusion pore kinetics in Ca(2+)-triggered exocytosis
- PMID: 20513396
- PMCID: PMC2877347
- DOI: 10.1016/j.bpj.2010.02.043
Membrane bending energy and fusion pore kinetics in Ca(2+)-triggered exocytosis
Abstract
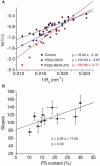
A fusion pore composed of lipid is an obligatory kinetic intermediate of membrane fusion, and its formation requires energy to bend membranes into highly curved shapes. The energetics of such deformations in viral fusion is well established, but the role of membrane bending in Ca(2+)-triggered exocytosis remains largely untested. Amperometry recording showed that during exocytosis in chromaffin and PC12 cells, fusion pores formed by smaller vesicles dilated more rapidly than fusion pores formed by larger vesicles. The logarithm of 1/(fusion pore lifetime) varied linearly with vesicle curvature. The vesicle size dependence of fusion pore lifetime quantitatively accounted for the nonexponential fusion pore lifetime distribution. Experimentally manipulating vesicle size failed to alter the size dependence of fusion pore lifetime. Manipulations of membrane spontaneous curvature altered this dependence, and applying the curvature perturbants to the opposite side of the membrane reversed their effects. These effects of curvature perturbants were opposite to those seen in viral fusion. These results indicate that during Ca(2+)-triggered exocytosis membrane bending opposes fusion pore dilation rather than fusion pore formation. Ca(2+)-triggered exocytosis begins with a proteinaceous fusion pore with less stressed membrane, and becomes lipidic as it dilates, bending membrane into a highly curved shape.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Han X., Wang C.T., Jackson M.B. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

