Human ehrlichiosis and anaplasmosis
- PMID: 20513551
- PMCID: PMC2882064
- DOI: 10.1016/j.cll.2009.10.004
Human ehrlichiosis and anaplasmosis
Abstract
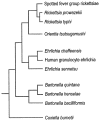
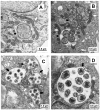
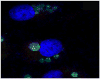
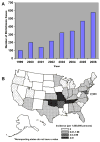
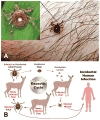
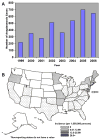
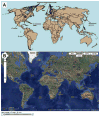
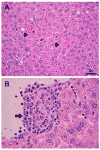
Human ehrlichiosis and anaplasmosis are acute febrile tick-borne diseases caused by various members of the genera Ehrlichia and Anaplasma (Anaplasmataceae). Human monocytotropic ehrlichiosis has become one of the most prevalent life-threatening tick-borne disease in the United States. Ehrlichiosis and anaplasmosis are becoming more frequently diagnosed as the cause of human infections, as animal reservoirs and tick vectors have increased in number and humans have inhabited areas where reservoir and tick populations are high. Ehrlichia chaffeensis, the etiologic agent of human monocytotropic ehrlichiosis (HME), is an emerging zoonosis that causes clinical manifestations ranging from a mild febrile illness to a fulminant disease characterized by multiorgan system failure. Anaplasma phagocytophilum causes human granulocytotropic anaplasmosis (HGA), previously known as human granulocytotropic ehrlichiosis. This article reviews recent advances in the understanding of ehrlichial diseases related to microbiology, epidemiology, diagnosis, pathogenesis, immunity, and treatment of the 2 prevalent tick-borne diseases found in the United States, HME and HGA.
2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. - PubMed
-
- Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. - PubMed
-
- Paddock CD, Liddell AM, Storch GA. Other causes of tick-borne ehrlichioses, including Ehrlichia ewingii. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-borne diseases of humans. ASM Press; Washington DC: 2005. pp. 258–267.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

