Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy
- PMID: 20513729
- PMCID: PMC2930982
- DOI: 10.1093/eurheartj/ehq136
Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy
Abstract
Aims: Hypertrophic cardiomyopathy (HCM) is an important cause of heart failure-related disability over a wide range of ages. Profiles of severe progressive heart failure symptoms and death, or heart transplantation deserve more complete definition within large patient cohorts.
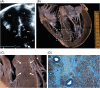
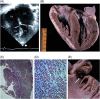
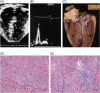
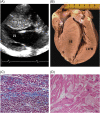
Methods and results: Clinical and morphological features of heart failure were assessed in 293 consecutive HCM patients over a median follow-up of 6 (inter-quartile range 2-11) years. Gross and histopathological features were analysed in 12 patients for whom the heart was available for inspection. Of the 293 patients, 50 (17%) developed severe progressive heart failure, including 18 who died or were transplanted. Three profiles of heart failure were identified predominantly associated with: (i) end-stage systolic dysfunction (ejection fraction <50%) (15; 30%); (ii) left ventricular (LV) outflow obstruction at rest (11; 22%); and (iii) non-obstructive with preserved systolic function (24; 48%). Overall, atrial fibrillation (AF) contributed to heart failure in 32 patients (64%) among the three profiles. Compared with other patients, those non-obstructive with preserved systolic function had earlier onset of heart failure symptoms mainly due to diastolic dysfunction, and the most accelerated progression to advanced heart failure and adverse outcome (P = 0.04). Thrombi were identified in the left atrial appendage of five gross heart specimens all belonging to patients with AF, including three of which were unrecognized clinically and had previously embolized. Extensive myocardial scarring with LV remodelling was evident in all end-stage patients; no or only focal scars were present in other patients.
Conclusion: Profiles of advanced heart failure in HCM are due to diverse pathophysiological mechanisms, including LV outflow obstruction and diastolic or global systolic ventricular dysfunction. Atrial fibrillation proved to be the most common disease variable associated with progressive heart failure. Recognition of the heterogeneous pathophysiology of heart failure in HCM is relevant, given the targeted management strategies necessary in this disease.
Figures
References
-
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113:1807–1816. doi:10.1161/CIRCULATIONAHA.106.174287. - DOI - PubMed
-
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi:10.1001/jama.287.10.1308. - DOI - PubMed
-
- Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi:10.1016/S0735-1097(00)01003-2. - DOI - PubMed
-
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi:10.1056/NEJM200006153422403. - DOI - PubMed
-
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. - PubMed

