Innate immunity defines the capacity of antiviral T cells to limit persistent infection
- PMID: 20513749
- PMCID: PMC2882831
- DOI: 10.1084/jem.20091193
Innate immunity defines the capacity of antiviral T cells to limit persistent infection
Abstract
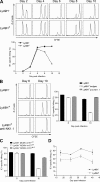
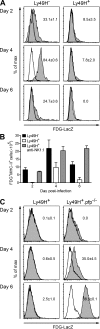
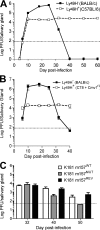
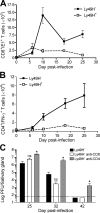
Effective immunity requires the coordinated activation of innate and adaptive immune responses. Natural killer (NK) cells are central innate immune effectors, but can also affect the generation of acquired immune responses to viruses and malignancies. How NK cells influence the efficacy of adaptive immunity, however, is poorly understood. Here, we show that NK cells negatively regulate the duration and effectiveness of virus-specific CD4+ and CD8+ T cell responses by limiting exposure of T cells to infected antigen-presenting cells. This impacts the quality of T cell responses and the ability to limit viral persistence. Our studies provide unexpected insights into novel interplays between innate and adaptive immune effectors, and define the critical requirements for efficient control of viral persistence.
Figures
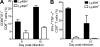


References
-
- Andoniou C.E., van Dommelen S.L., Voigt V., Andrews D.M., Brizard G., Asselin-Paturel C., Delale T., Stacey K.J., Trinchieri G., Degli-Esposti M.A. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 6:1011–1019 10.1038/ni1244 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

